|
Case Report
Hirschsprung disease of the colon, a vaginal mass and medullary thyroid cancer – a RET oncogene driven problem
Romy Pandey1, Tiffany Thurow2, Robert de W Marsh3
1Departments of Internal Medicine; 2Pathology, North Shore University Health System and University of Chicago Pritzker School of Medicine, Chicago;
3Department of Gastrointestinal Oncology, North Shore University Health System, Evanston, Illinois, USA
Corresponding author: Dr Robert de W Marsh. Department of Gastrointestinal
Oncology, 2650 Ridge Ave, Evanston, Illinois 60201, United States. Tel:
(847) 570-2112; Fax: (847) 570-2336. Email: rmarsh@northshore.org
|
|
Abstract
This case report emphasizes the fact that all patients with Hirschsprung disease should be screened for RET Oncogene
mutation as there is a well known association between Hirschsprung Disease and Multiple Endocrine Neoplasia (MEN)
Type 2A. It also reminds us that Medullary Thyroid Carcinoma is known to cause elevated levels of CEA which does not
originate from gastrointestinal tract.
Key words
Hirschsprung Disease, RET Oncogene, Medullary Thyroid Cancer
J Gastrointest Oncol 2011; 2: 254-257. DOI: 10.3978/j.issn.2078-6891.2011.028
|
|
Case Report
A 55 year old postmenopausal woman presented with
vaginal spotting which rapidly progressed to more severe
bleeding.
On examination she was found to have a mass in the
vaginal vault which was close to, but not attached to, the
cervix. Excisional biopsy of the lesion in the vaginal wall
and biopsies of the endometrium, along with cervical
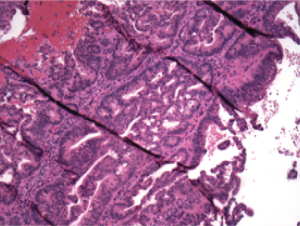
conization revealed adenocarcinoma in the vaginal lesion
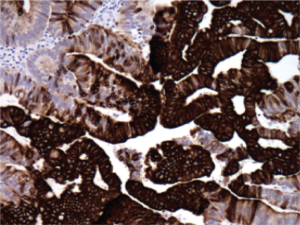
only ( Figure 1). Immunostains were performed and these
showed a pattern which was most compatible with intestinal
differentiation (CK20 and CDX-2 positive, CK7 focally
positive (less than 5% of cells), ER and PR both negative,
P16 and CEA variably positive) ( Figure 2). The Tissue of
Origin Test®, run on micro dissected tumor tissue, showed
the highest similarity score of 91.1 for a colo-rectal origin.
The 14 other tissue types in the panel had similarity scores
of less than or equal to 5. CT scan and MRI of the abdomen
and pelvis showed several cavernous hemangiomas and cysts in the liver but there was no evidence of any residual
or metastatic disease. PET scan was also unremarkable.
Additional history of Hirschprung disease (HD) as a child,
which had required surgical correction (with complications
of obstruction and fistula formation at age 19 which were
addressed with additional surgery), was obtained. Anorectal
examination was grossly unremarkable and random biopsies
showed mucosa consistent with a squamous papilloma but
with no evidence of malignancy. Colonoscopy was normal.
Of note, her CEA level at this time was found to be elevated
at 35 ng/ml (normal range 0-5 ng/ml). Family history was significant for colorectal cancer in her
mother and grandfather and endometrial and appendiceal
cancer in a cousin. Her brother had also been treated for
HD.
At this time she was referred to medical oncology.
Physical examination, including a pelvic exam and
careful exam of the thyroid, was unremarkable. A repeat
PET scan now showed slightly irregular, moderately
increased radionuclide accumulation in both lobes of the
thyroid. Ultrasound showed a diffusely heterogeneous
gland mimicking conf luent nodules. TSH was normal
but unstimulated serum calcitonin was elevated at 121
pg/mL (Reference Value - Basal: <8) and CEA remained
abnormal but stable at 37 ng/ml. Ultrasound guided fine
needle biopsy of the thyroid was consistent with medullary
thyroid carcinoma (MTC). As a result, she underwent total
thyroidectomy and paratracheal lymph node dissection. A
22 gram thyroid revealed a 1.5 cm yellow-tan, firm nodule
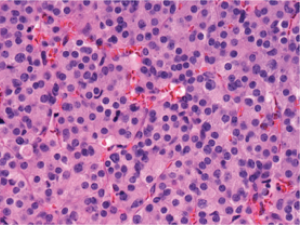
in the left superior lobe and a 0.7 cm yellow-tan, firm nodule in the right inferior lobe. Histologic examination of each
of the nodules revealed sheets and nests of monomorphic
cells with abundant granular cytoplasm and uniform nuclei
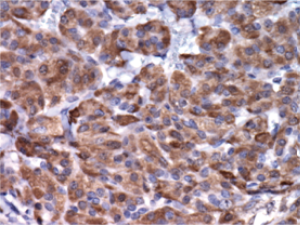
with stippled chromatin ( Figure 3). Immunohistochemical
evaluation of these cells revealed positive staining with
calcitonin (Figure 4), chromogranin, and synaptophysin.
Staining was negative with thyroglobulin. There was no
lymph node involvement. The diagnosis of T1b N0 MTC
was thus confirmed. Both CEA and calcitonin levels normalized following surgery. A subsequent evaluation for MEN (Multiple Endocrine
Neoplasia) syndrome included a 24-hour urine collection
for met anephr ine and normet anephr ine and bot h
metanephrine 47 mcg/24hrs (reference range 30-180
mcg/24hrs) and normetanephrine 126 mc/24hrs (reference
range 128 -484 mcg/24 hrs) were found to be normal.
Despite these normal findings, a high suspicion for RET
oncogene mutation persisted, given her history of MTC as well as a history of HD, with the result that genetic
consultation was requested.
Following appropriate counseling, she was tested and
found to be positive for a specific RET mutation, C620W,
diagnostic of MEN2A. Her sister then also tested positive
for the same RET mutation. This particular mutation is
known to be associated with familial HD, but in contrast to
other RET gene mutations, is less strongly correlated with
parathyroid and adrenal disease.
She has continued to have physical examination,
blood tests and serial imaging in follow up, and thus far
there has been no evidence of recurrent or new disease.
The origin of the adenocarcinoma in the vaginal vault is
still unclear. Given the definitive diagnosis of medullary
thyroid carcinoma, immunohistochemical staining for
calcitonin was performed on the tumor cells and was
negative. Therefore, a diagnosis of adenocarcinoma
of unknown origin remains and any relationship to
the MEN syndrome or the RET germline mutation is
undefined. Continued surveillance for a possible primary
site continues.
|
|
Discussion
Germline mutation of the RET (REarranged during
Transfection) proto-oncogene (10q 11.2) may result
in constitutively activated RET protein. RET protein
consists of an extracellular ligand-binding domain, a
transmembrane domain, and an intracellular domain,
which contains two tyrosine kinase subdomains (TK1
and TK2) that are involved in the activation of several
intracellular signal transduction pathways. There is
a correlation between specific mutations and specific
disease phenotypes ( 1). Mutations in RET exons 10
(codons 609, 611, 618, and 620) or 11 (codons 630
or 63 4), a re seen in the major it y of MEN2A and
FMTC (Familial medullary thyroid cancer) ca ses
resulting in alterations in the cysteine-rich region of
the RET protein’s extracellular domain. A mutation
in codon 634 in exon 11 is the most common genetic
defect in this disorder and is strongly associated with
hyperparathyroidism and pheochromocytoma (PC) in
MEN2A. Mutations in codons 768 (exon 13), 804 (exon
14) and 891 (exon 15), which result in changes in the
intracellular tyrosine kinase domains, are found only in
FMTC ( 2). In MEN 2B patients, the mutation involves
codon 918 in e xon 16 in 95% of ca ses and, ra rely,
codon 883 in exon 15 with resultant change in either
methionine or alanine, respectively, in the tyrosine
kinase domain of RET ( 3). Germaine to our patient and her family, in the rare cases where MEN 2A and HD co-exist, germline RET mutations
most often involve exon 10 ( 1, 4), especially codon 618 or
620 ( 1, 5). This association poses a scientific dilemma, as the
mutations in MEN are gain of function mutations with RET
acting as a dominantly acting oncogene ( 6, 7) and those of
HD result in loss of function ( 8, 9). However, a unifying
hypothesis has been suggested in that mutations in exon 10
result in a relatively weaker activation of the RET protein
kinase, perhaps just sufficient to cause MTC. A concurrent
decrease in the total number of receptor molecules on
the cell surface possibly results in insufficient numbers of
receptors for normal gangliogenesis and migration and/or
for the prevention of inappropriate apoptosis, with HD as a result ( 10, 11). This case teaches us a number of important lessons. Firstly, that all patients with a history of HD should consider screening for RET mutations (it should be noted that RET mutations are the predominant but only one of a number of possible causes of HD) ( 12, 13), asthere is a well established association between HD and MEN2A. If present, this could facilitate early diagnosis of MEN2A with resultant thyroidectomy prior to the onset of MTC or at least pr ior to the development of metastatic disease. Equally, it is desirable that all patients with MTC should be tested for germline RET mutations in accordance with 2009 American Thyroid Association Guidelines for Management of MTC ( 14). While somatic mutation of RET gene are limited to C cells with no additional risk of neoplasia in other tissues (approximately 50% of patients with sporadic MTC have somatic RET mutations), germline mutations affect all the tissues derived from neural crest such as neural cells, neuroendocrine cells and urogenital cells, causing MEN syndromes and rarely HD. Secondly, it reminds us that MTC is a potential cause of elevated CEA which does not have its origins in bowel cancer. Unlike calcitonin levels, which are susceptible to stimulation and hence tend to f luctuate on serial measurements, CEA levels are more stable and can be used as a tumor marker for MTC. Elevated CEA levels have been associated with increased tumor aggressiveness, tumor recur rence, and poor prognosis. Thirdly, it illustrates the value of a thorough genetic evaluation in all patients suspected of having a genetic component to their disease. This could have profound implications not only for the index patient but also for family members. Finally, it reconfirms the value of a good history and physical examination, and the therapeutic challenges presented by cancer of unknown origin, even with the sophist icated genome based diagnostics available today ( 15).
|
|
References
- Eng C, Clayton D, Schuffenecker I, Lenoir G, Cote G, Gagel RF, et al.
The relationship between specific RET proto-oncogene mutations and
disease phenotype in multiple endocrine neoplasia type 2. International
RET mutation consortium analysis. JAMA 1996;276:1575-9.[LinkOut]
- Margraf RL, Crockett DK, Krautscheid PM, Seamons R, Calderon
FR, Wittwer CT, et al. Multiple endocrine neoplasia type 2 RET
protooncogene database: repositor y of MEN2-associated RET
sequence variation and reference for genotype/phenotype correlations.
Hum Mutat 2009;30:548-56.[LinkOut]
- Gimm O, Marsh DJ, Andrew SD, Frilling A, Dahia PL, Mulligan LM,
et al. Germline dinucleotide mutation in codon 883 of the RET protooncogene
in multiple endocrine neoplasia type 2B without codon 918
mutation. J Clin Endocrinol Metab 1997;82:3902–4.[LinkOut]
- Decker RA, Peacock ML, Watson P. Hirschsprung disease in MEN 2A:
increased spectrum of RET exon 10 genotypes and strong genotypephenotype
correlation. Hum Mol Genet 1998;7:129–34.[LinkOut]
- Caron P, Attié T, David D, Amiel J, Brousset F, Roger P, et al. C618R
mutation in exon 10 of the RET proto-oncogene in a kindred with
multiple endocrine neoplasia type 2A and Hirschsprung's disease. J
Clin Endocrinol Metab 1996;81:2731–3.[LinkOut]
- Asai N, Iwashita T, Matsuyama M, Takahashi M. Mechanism of
activation of the ret proto-oncogene by multiple endocrine neoplasia 2A
mutations. Mol Cell Biol 1995;15:1613–9.[LinkOut]
- Santoro M, Carlomagno F, Romano A, Bottaro DP, Dathan NA,
Grieco M, et al. Activation of RET as a dominant transforming gene by
germline mutations of MEN2A and MEN2B. Science 1995;267:381–3.[LinkOut]
- Pasini B, Borrello MG, Greco A, Bongarzone I, Luo Y, Mondellini P,
et al. Loss of function effect of RET mutations causing Hirschsprung
disease. Nat Genet 1995;10:35–40.[LinkOut]
- Pelet A, Geneste O, Edery P, Pasini A, Chappuis S, Atti T, et al. Various
mechanisms cause RET-mediated signaling defects in Hirschsprung's
disease. J Clin Invest 1998;101:1415–23.[LinkOut]
- Eng C. RET proto-oncogene in the development of human cancer. J
Clin Oncol 1999;17:380-93.[LinkOut]
- Arighi E, Popsueva A, Degl'Innocenti D, Borrello MG, Carniti C, Perälä
NM, et al. Biological effects of the dual phenotypic Janus mutation of
ret cosegregating with both multiple endocrine neoplasia type 2 and
Hirschsprung's disease. Mol Endocrinol 2004;18:1004-17.[LinkOut]
- Parisi MA, Kapur RP. Genetics of Hirschsprung disease. Curr Opin
Pediatr 2000;12:610-7.[LinkOut]
- Martucciello G, Ceccherini I, Lerone M, Jasonni V. Pathogenesis of
Hirschsprung's disease. J Pediatr Surg 2000;35:1017-25.[LinkOut]
- American Thyroid Association Guidelines Task Force, Kloos RT, Eng
C, Evans DB, Francis GL, Gagel RF, et al. Medullary thyroid cancer:
management guidelines of the American Thyroid Association. Thyroid
2009;19:565-612.[LinkOut]
- Pillai R, Deeter R, Rigl CT, Nystrom JS, Miller MH, Buturovic L, et al.
Validation and reproducibility of a microarray-based gene expression
test for tumor identification in formalin-fixed, paraffin-embedded
specimens. J Mol Diagn 2011;13:48-56.[LinkOut]
Cite this article as: Pandey R, Thurow T, Marsh R. Hirschsprung disease of the colon, a vaginal mass and medullary thyroid cancer – a RET oncogene driven problem. J Gastrointest Oncol. 2011;2(4):254-257. DOI:10.3978/j.issn.2078-6891.2011.028
|