Pencil beam scanning versus passively scattered proton therapy for unresectable pancreatic cancer
Introduction
A major consideration when treating locally advanced pancreatic cancer (LAPC) with radiation therapy is potentially serious toxicity caused by unintentional dose to radiosensitive organs at risk (OARs) including the small bowel, stomach, kidneys, and liver. This is despite the use of modern photon therapy techniques (1,2). As a result, the prescription dose is typically limited to ~50–54 in 1.8–2.0 Gy fractions, which is suboptimal for treatment of gross disease. Attempts at significant dose escalation with photon therapy have largely not been successful due to increased toxicity (3-5) with the exception of some patients with favorable anatomy (6,7).
Proton therapy is expected to improve the therapeutic ratio for some patients because of its lack of exit dose. Published experiences suggest that using proton can both reduce the incidence of grade 3–4 gastrointestinal (GI) adverse effects beyond what is achievable with photon therapy as well as allow for safe dose escalation and/or chemotherapy intensification in patients with pancreatic cancer (6,8-12). For example, a phase 2 LAPC trial from the University of Florida Proton Therapy Institute (UFPTI) that prescribed 59.4 Gy [relative biological effectiveness (RBE)] in 1.8 Gy (RBE) fractions with concurrent capecitabine reported no grade 2 or higher GI toxicities despite the use of radiation dose escalation (13). These encouraging results may be the result of significant small bowel and stomach sparing especially in the lower dose range that otherwise would not be possible using photons; the small bowel volume that receives low-to-moderate radiation dose is an important predictor of GI toxicity (14-17).
Most dosimetric and clinical studies of proton therapy for pancreas cancer have used passive scattering (PS), in which a narrow proton beam is scattered over a larger area to adequately treat the entire target volume. While PS provides excellent dose conformality distal to the target, it lacks a high level of proximal dose conformality (18). Pencil beam scanning (PBS), in which a proton beam is magnetically scanned across the target volume, can achieve both distal and proximal dose conformality thus potentially further improving the therapeutic ratio (9,12).
Because of the increasing clinical evidence showing the benefit of PBS for various malignancies such as those of the skull base (19-21) we were interested in identifying whether PBS may also benefit pancreatic cancer patients. Therefore, we performed a dosimetric comparison of PBS and PS to better understand the possible clinical applications of PBS for LAPC. We hypothesized that PBS would provide a significant benefit in OAR sparing because of its three-dimensional conformality, especially with respect to the duodenum that is proximal to the pancreas when using posterior and right lateral beams.
Methods
This study was performed after Institutional Review Board (IRB) approval was obtained from both contributing institutions—UFPTI and University of Maryland Medical Center (UMMC).
PBS proton plans were retrospectively generated at UMMC for LAPC patients originally treated with PS proton therapy (PSPT) on a phase 2 trial at UFPTI; the treatment planning details and outcomes of this trial have been published (13). Briefly, 59.4 Gy (RBE) in 1.8 Gy (RBE) daily fractions was prescribed to the PTV with the prescription dose being required to cover at least 95% of the PTV. The average scan from the planning 4-dimensional computed tomography (4DCT) was used for planning. Density override with water equivalent density was done for bowel gas and verification plans were done on the original non-overridden scan to ensure target coverage. The primary tumor only was targeted and elective nodal irradiation was not performed. Motion management strategies such as breath hold, respiratory gating, or abdominal compression were not employed. 4DCT simulation was performed from which an internal gross tumor volume (IGTV) was created. The internal clinical target volume (ICTV) was defined as the IGTV plus 3–10-mm expansion. For the PS plans the planning target volume (PTV) was defined as the ICTV plus 5–10-mm expansion. PS plans required apertures for lateral dose distribution conformity and range compensators for distal dose distribution conformity.
Anonymized CT images, OAR contours, and target volume contours for all patients treated on the UFPTI trial were sent to a blinded planning team at UMMC, which generated PBS proton plans using the Eclipse treatment planning system (TPS) (Palo Alto, California, USA). The PBS planning team was instructed to use the identical prescription dose, target coverage goals, OAR dose objectives (Table 1), and treatment couch used for PS plans. The PBS planning team was also instructed to use a more heavily weighted posterior-oriented beam and a right lateral-oriented beam, as was done on the UFPTI trial. These beam angles were selected to minimize small bowel dose while simultaneously minimizing uncertainty from organ motion and filling. As was done for PS plans, bowel gas density override was used in PBS planning and verification plans were done on the original non-overridden scan to ensure target coverage. Robust plan analyses were run on the ICTV with respect to at least 95% of the prescription dose covering at least 95% of the ICTV.
Full table
For PBS plans, proximal/distal PTV margins were based on 3% Hounsfield unit density uncertainty plus 1 mm, plus an additional 2–3 mm to account for bowel gas, as needed, to a maximum of 1.1 cm. All PBS plans used single field uniform dose optimization (SFUD) to improve robustness. Robustness analysis was performed on 12 scenarios by shifting ±5 mm in X, Y, and Z directions while assuming a proton stopping power of ±3.5%. PBS planning included the assumption that a minimum of 3 monitor units (MU) per spot could be delivered. Because the Eclipse TPS does not account for differential beam weighting until after optimization, applying beam weighting after optimizing resulted in some spots in the right lateral field to fall below 3 MU per spot in post-processing. To account for this, a range shifter was used for the right lateral fields in patients where more extreme weighting was used in the posteroanterior (PA) field (e.g., 75% posterior, 25% right lateral) to create a more homogeneous dose distribution and to be able to meet target volume constraints.
A Wilcoxon t-test was used to compare various dosimetric parameters for normal organs and target volumes between proton delivery techniques (PBS vs. PS).
Results
Target volume
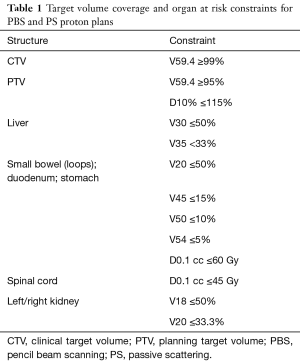
The median ICTV and PTV volumes were 136.7 cc (range, 69.3–225.9 cc) and 256.6 cc (range, 72.3–440.7 cc), respectively. The PBS plans achieved all target volume coverage goals, but the PTV coverage goal (V100% >95%) was not met in 1 of 11 PS plans (range, 81.8–98.9%). In addition, the median PTV volume receiving at least the prescription dose [V59.4 (RBE)] was higher with PBS [97% (range, 95.9–100.0%) vs. 95.1% (range, 81.8–98.9%); P=0.001], as shown in Figure 1. PBS plans were also cooler in the high-dose range; the median relative prescription dose received by at least 10% of the PTV (D10%) was 102.4% (range, 101.6–103.2%) compared to 103.8% (range, 102.8–105.9%) using PS (P=0.001). A trend towards higher ICTV V59.4 favored PBS [100% (range, 98.1–100%) vs. 98.8% (range, 92.0–100.0%); P=0.070] (Figure 1).
Small bowel
There was no significant difference between PBS and PS plans in median small bowel V5 Gy (RBE), V10 Gy (RBE), V15 Gy (RBE), V20 Gy (RBE), V25 Gy (RBE), V30 Gy (RBE), V35 Gy (RBE), V40 Gy (RBE), V45 Gy (RBE), V50 Gy (RBE) [V5 Gy (RBE)…V50 Gy (RBE)], or V54 Gy (RBE). There was a small absolute, but statistically significant difference in median V59.4 Gy (RBE) favoring PBS (0.11% vs. 0.37%; P=0.012) (Figure 1).
Duodenum
There was no significant difference between PBS and PS plans in median duodenum V5 Gy (RBE)…V50 Gy (RBE), or V54 Gy (RBE). There was a small absolute, but statistically significant difference in median V59.4 Gy (RBE) favoring PBS (37.4% vs. 40.4%; P=0.014) (Figure 1).
Stomach
There was no significant difference between PBS and PS plans in median stomach V5 Gy…V50 Gy (RBE), or V54 Gy (RBE). There was a small absolute, but statistically significant difference in median V59.4 Gy (RBE) favoring PBS (0.01% vs. 0.1%; P=0.023) (Figure 1).
Liver
Liver dose was higher in the PBS plans in median V5 Gy (RBE) (24.1% vs. 20.2%; P=0.032), V20 Gy (RBE) (3.2% vs. 2.8%; P=0.010), and V25 Gy (RBE) (2.6% vs. 2.2%; P=0.019). No significant difference was found for median V10 Gy (RBE), V15 Gy (RBE), V30 Gy (RBE), V35 Gy (RBE), or mean dose (Figure 1).
Kidneys
No significant difference was found in median left or right kidney V18 Gy (RBE), V20 Gy (RBE), or V25 Gy (RBE). No significant difference was found between median combined left/right kidney V12 Gy (RBE) or mean dose (Figure 1).
Spinal cord
There was no significant difference in spinal cord dose with the exception of lower median absolute dose at 0.1 cc in PBS plans [35.7 vs. 39.4 Gy (RBE); P=0.019] (Figure 1).
Discussion
Compared to the distal conformality of PSPT, PBS provides three-dimensional conformality particularly in high dose regions. As such, PBS has increasingly become used to treat certain cancers, especially those in challenging anatomic locations including the base of skull (19), head and neck, and lung (20). Cancers of the pancreas are also especially challenging to treat given the intimate proximity to radiosensitive GI luminal structures like the duodenum. While there are data supporting the use of PSPT for these patients, potential benefits of the more highly conformal PBS technique are not well described.
Thompson et al. published the only comparison of PBS and PSPT for LAPC and reported that PBS offered better sparing in the low and moderate dose ranges for multiple OARs including the duodenum and small bowel (9). The extent of small bowel dose reduction in their study was modest, and while the volume of small bowel receiving lower dose is an important predictor of GI toxicity, the clinical implications of their results are uncertain (14-16). Neither they nor we performed normal tissue complication probability analyses. There are some notable differences between their study and ours: (I) while a single dosimetrist generated all plans in their study we used blinded PBS and PS teams to decrease planning bias, and (II) we used a coplanar posterior and right lateral-oriented 2-beam approach while they used a noncoplanar 3-beam technique, which they selected to specifically minimize duodenal dose from the beam penumbra (22).
Our study demonstrated that PBS improved some aspects of target volume coverage, such as volume of PTV receiving prescription dose and decreased hot spots compared to PS as hypothesized. Additionally, we hypothesized that the conformality of PBS would be beneficial in minimizing dose to various OARs, but especially the duodenum that lies immediately proximal to the pancreas when using a posterior and right lateral-oriented beam arrangement. We did in fact find that PBS plans delivered a lower duodenal median V59.4 Gy (RBE) (37.4% vs. 40.4%; P=0.014), but otherwise did not detect any significant differences at lower doses. While statistically significant differences in sparing of the small bowel, stomach, and spinal cord favored PBS, the absolute reductions were small. This coupled with the fact that all plans, regardless of proton delivery technique, had no difficulty meeting OAR constraints these differences many have minimal if any clinical relevance although additional studies are needed for clarification. Interestingly, the volume of the liver receiving lower dose [e.g., V5 Gy (RBE)] was actually higher in PBS plans, which was not expected given that PBS plans are typically more conformal.
Several important methodological factors may have limited our ability to detect larger OAR sparing differences between PBS and PS. First, patients were treated with a GTV-to-PTV expansion of up to 20 mm to account for both tumor motion (no motion management was used) and setup uncertainty; this led to significant PTV overlap of the duodenum for most patients as illustrated by the median duodenal dose being the prescription dose of 59.4 Gy (RBE) (Figures 1,2). The overlap likely reduced our ability to identify any benefits of PBS in duodenal sparing for the majority of the patients in our study. Of note, Thompson et al. used smaller 10-mm PTV expansions by assuming the use of breath hold, which likely led to reduced duodenal overlap by the PTV. Second, while we required similar beam angles to be used for PBS and PS plans we did not mandate use of equal beam weighting. While 25% right lateral weighting was used for every PS plan, the right lateral beams were generally more heavily weighted in PBS plans: 25% in 6 plans (55%), 30% in 2 plans (18%), and 40% in 3 PBS plans (27%). Therefore, the dose received by OARs in the path of the right lateral beam, including the duodenum, could have been influenced by differences in beam weighting. This is also a plausible explanation for why the liver received higher dose in PBS plans. Lastly, the use of a range shifter for the right lateral PBS beam in approximately half of the patients may have also contributed to increasing dose to OARs within the beam path as a result of larger spot size and a broader beam penumbra.
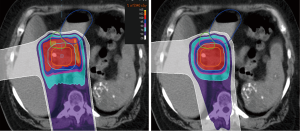
We highlight that this analysis was purely a dosimetric comparison and that we cannot conclude what is the clinical significance of our data. Furthermore, our analysis does not reflect the effect of inhomogeneities within the beam path on delivered versus intended dose distribution as a result of motion; these effects are expected to be greater for PBS, which is less robust than PS. Finally, because PBS has greater advantages for treating targets with more complex and irregular shapes we suspect that larger differences would be identified if the primary tumor and elective lymph nodes were both targeted instead of the primary pancreas tumor alone as was done in this study (20).
In conclusion, despite treatment planning differences that may have limited our ability to detect a larger benefit of PBS, PBS can deliver at least equivalent dose to at least most OARs while improving target volume coverage for LAPC patients. Additional studies are needed to clarify which pancreatic cancer patient subsets may derive the greatest benefit from PBS compared to PSPT.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical statement: This study was performed after Institutional Review Board (IRB) approval was obtained from both contributing institutions—University of Florida Proton Therapy Institute (UFPTI) (No. UFJ2014-224) and University of Maryland Medical Center (No. IRB00000233).
References
- Loehrer PJ Sr, Feng Y, Cardenes H, et al. Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 2011;29:4105-12. [Crossref] [PubMed]
- Bittner MI, Grosu AL, Brunner TB. Comparison of toxicity after IMRT and 3D-conformal radiotherapy for patients with pancreatic cancer - a systematic review. Radiother Oncol 2015;114:117-21. [Crossref] [PubMed]
- McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2001;19:4202-8. [Crossref] [PubMed]
- Ben-Josef E, Schipper M, Francis IR, et al. A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2012;84:1166-71. [Crossref] [PubMed]
- Ceha HM, van Tienhoven G, Gouma DJ, et al. Feasibility and efficacy of high dose conformal radiotherapy for patients with locally advanced pancreatic carcinoma. Cancer 2000;89:2222-9. [Crossref] [PubMed]
- Bouchard M, Amos RA, Briere TM, et al. Dose escalation with proton or photon radiation treatment for pancreatic cancer. Radiother Oncol 2009;92:238-43. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Nichols RC, George TJ, Zaiden RA, et al. Proton therapy with concomitant capecitabine for pancreatic and ampullary cancers is associated with a low incidence of gastrointestinal toxicity. Acta Oncologica 2013;52:498-505. [Crossref] [PubMed]
- Thompson RF, Mayekar SU, Zhai HF, et al. A dosimetric comparison of proton and photon therapy in unresectable cancers of the head of pancreas. Medical Physics 2014;41. [Crossref] [PubMed]
- Hsiung-Stripp DC, McDonough J, Masters HM, et al. Comparative treatment planning between proton and X-ray therapy in pancreatic cancer. Medical Dosimetry 2001;26:255-9. [Crossref] [PubMed]
- Nichols RC Jr, Huh SN, Prado KL, et al. Protons offer reduced normal-tissue exposure for patients receiving postoperative radiotherapy for resected pancreatic head cancer. Int J Radiat Oncol Biol Phys 2012;83:158-63. [Crossref] [PubMed]
- Ding X, Dionisi F, Tang S, et al. A comprehensive dosimetric study of pancreatic cancer treatment using three-dimensional conformal radiation therapy (3DCRT), intensity-modulated radiation therapy (IMRT), volumetric-modulated radiation therapy (VMAT), and passive-scattering and modulated-scanning proton therapy (PT). Med Dosim 2014;39:139-45. [Crossref] [PubMed]
- Sachsman S, Nichols RC Jr, Morris CG, et al. Proton Therapy and Concomitant Capecitabine for Non-Metastatic Unresectable Pancreatic Adenocarcinoma. Int J Part Ther 2014;1:692-701. [Crossref]
- Huang J, Robertson JM, Ye H, et al. Dose-volume analysis of predictors for gastrointestinal toxicity after concurrent full-dose gemcitabine and radiotherapy for locally advanced pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys 2012;83:1120-5. [Crossref] [PubMed]
- Olsen JR, Moughan J, Myerson RJ, et al. Predictors of radiotherapy-related gastrointestinal toxicity from anal cancer DP-IMRT: Secondary analysis of RTOG 0529. J Clin Oncol 2014;90:S31.
- Baglan KL, Frazier RC, Yan D, et al. The dose-volume relationship of acute small bowel toxicity from concurrent 5-FU-based chemotherapy and radiation therapy for rectal cancer. Int J Radiat Oncol Biol Phys 2002;52:176-83. [Crossref] [PubMed]
- Gunnlaugsson A, Kjellen E, Nilsson P, et al. Dose-volume relationships between enteritis and irradiated bowel volumes during 5-fluorouracil and oxaliplatin based chemoradiotherapy in locally advanced rectal cancer. Acta Oncologica 2007;46:937-44. [Crossref] [PubMed]
- Torres MA, Chang EL, Mahajan A, et al. Optimal treatment planning for skull base chordoma: photons, protons, or a combination of both? Int J Radiat Oncol Biol Phys 2009;74:1033-9. [Crossref] [PubMed]
- Grosshans DR, Zhu XR, Melancon A, et al. Spot scanning proton therapy for malignancies of the base of skull: treatment planning, acute toxicities, and preliminary clinical outcomes. Int J Radiat Oncol Biol Phys 2014;90:540-6. [Crossref] [PubMed]
- Zhang X, Li Y, Pan X, et al. Intensity-modulated proton therapy reduces the dose to normal tissue compared with intensity-modulated radiation therapy or passive scattering proton therapy and enables individualized radical radiotherapy for extensive stage IIIB non-small-cell lung cancer: a virtual clinical study. Int J Radiat Oncol Biol Phys 2010;77:357-66. [Crossref] [PubMed]
- Frank SJ, Cox JD, Gillin M, et al. Multifield optimization intensity modulated proton therapy for head and neck tumors: a translation to practice. Int J Radiat Oncol Biol Phys 2014;89:846-53. [Crossref] [PubMed]
- Chang DS, Bartlett GK, Das IJ, et al. Beam angle selection for intensity-modulated radiotherapy (IMRT) treatment of unresectable pancreatic cancer: are noncoplanar beam angles necessary? Clin Transl Oncol 2013;15:720-4. [Crossref] [PubMed]


