Neoadjuvant PET and MRI-based intensity modulated radiotherapy leads to less toxicity and improved pathologic response rates in locally advanced rectal cancer
Introduction
Neoadjuvant chemoradiation (NeoCRT) for the treatment of clinical T3/4 or lymph node positive locally advanced rectal cancer (LARC) improves rates of sphincter preservation and local control while reducing the risk for acute and long term toxicities (1). Despite these advances, occurrences of grade 2 and 3 gastrointestinal (GI) toxicities with NeoCRT remain high and have been shown to have negative impacts on quality of life (2) and sexual function (3). Relative to 3 dimensional conformal radiotherapy (3DCRT), intensity modulated radiotherapy (IMRT) allows for a more conformal dose distribution resulting in lower dose to organs at risk (OAR) (4) with improved target coverage (5). Additionally, advanced pre-treatment imaging, such as 18F fluorodeoxyglucose (FDG) positron emission tomography (PET) and magnetic resonance imaging (MRI), may lead to stage migration through identification of new lesions not seen on diagnostic computed tomography (CT) imaging, as well as improved delineation of radiotherapy target volumes (6,7). In spite of these advances, no impact on clinical outcomes has been seen.
Utilization of IMRT has been prospectively explored in the treatment of pelvic malignancies, such as anal Cervical and endometrial cancers (8,9). In these studies, use of IMRT led to a reduction in adverse GI toxicities compared to conventional 3DCRT, yet experiences in the treatment of rectal cancers with IMRT remain sparse with conflicting results. Whereas retrospective series have reported reduced rates of acute GI toxicities with IMRT in rectal cancer, these have not led to improvements in clinical outcomes, such as pathologic complete response (pCR) and local control rates unlike benefits seen in other malignancies (10). The single arm combination of neoadjuvant IMRT with non-standard capecitabine and oxaliplatin in the Radiotherapy Oncology Group (RTOG) 0822 (11) did not significantly reduce rates of grade ≥2 GI toxicities when compared to RTOG 0247 (12) which used 3DCRT with concurrent capecitabine and oxaliplatin. In spite of this, comparable pCR rates suggested that tumor coverage was not compromised by improving conformity of radiotherapy. Herein, we report a contemporary multi-institutional series on the use of IMRT and advanced pre-treatment imaging in the neoadjuvant treatment of rectal cancer and resulting impact on toxicities and pathologic outcomes relative to 3DCRT.
Methods
Retrospective identification of 128 patients with LARC from 2007–2016 at 4 major academic institutions was performed with Institutional Review Board approval. Patients treated with neoCRT using 3DCRT or IMRT followed by definitive oncologic resection were selected for further study. All patients had biopsy proven adenocarcinoma of the rectum and underwent initial staging with CT and/or pelvic MRI, endorectal ultrasonography (ERUS) or PET. Patients were treated by radiation oncologists who specialize in the treatment of GI malignancies. Patients received CT-based simulation using VacLoc or equivalent for pelvic immobilization, anal marker placement, and intravenous and oral contrast unless medically contraindicated. All treated with 3DCRT or IMRT were simulated either prone with belly board or supine. The RTOG Anorectal Contouring Atlas was utilized as a guide for delineation of treatment volumes (13). The gross tumor volumes (GTV) comprised primary tumor and involved regional lymph nodes identified clinically and/or radiographically. Initial clinical target volumes (CTV) included primary and nodal GTVs, as well as mesorectal, presacral and internal iliac nodes. For cT4 tumors, external iliac lymph nodes were included. For primary tumors at or below the dentate line, external iliac and inguinal lymph node basins were included. The boost CTV included primary rectal GTV (1.5–2 cm radial and 2.5–3 cm craniocaudal expansions) and nodal GTV (1 cm isotropic expansion). Planning target volume (PTV) expansions of 0.5–1 cm were added to CTVs. Initial and boost PTVs were prescribed 4,500 cGy and 540–900 cGy in 180 cGy fractions. 3DCRT treatment plans utilized opposed laterals and PA beams, and IMRT treatments were inversely planned. Normal tissue constraints from RTOG 0822 were utilized (11). Image guidance with weekly MV or daily cone-beam CT was performed. All patients received concurrent continuous infusion intravenous 5-FU (225 mg/m2) or oral capecitabine (825 mg/m2 twice daily on the days of radiotherapy. Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v 4.0) were used to grade toxicities on a 5-point scale prior to treatment and weekly during treatment. Patient, disease and treatment specific characteristics including, but not limited to gender, age, body mass index (BMI), type of pre-treatment diagnostic imaging, Karnofsky performance status (KPS), race, location from the anal verge, radiation dose, treatment delays, clinical and pathologic staging and response were obtained. Following chemoradiation, patients underwent total mesorectal excision via lower anterior resection or abdominoperineal resection. Degree of pathologic response (PR) was reported at each institution as progressive disease, no response, any partial response or complete response.
Statistical analysis
Baseline characteristics were compared across radiation types (IMRT vs. 3DCRT) using Chi-square test for categorical variables, and Wilcoxon rank-sum test for continuous variables. Univariable analyses were performed for each acute toxicity. Multivariable logistic regression models were fit to evaluate the association between radiation type and the occurrence of any serious adverse event (grade >2 vs. ≤2). Logistic regression models were also fit to evaluate the association between partial or complete PR versus no response. All models were adjusted for age, sex, and pre-treatment chemotherapy.
Results
Clinical characteristics
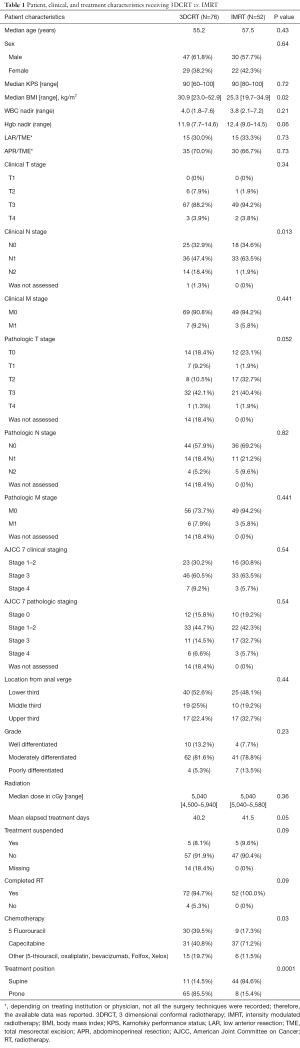
Of the 128 patients treated with NeoCRT, 76 patients (59.4%) received 3DCRT and 52 patients (40.6%) received IMRT. T3 disease and node positivity were identified in 90.6% and 65.6% of patients, respectively. Radiation doses ranged from 4,500–5,940 cGy (median dose 5,040 cGy). Baseline patient characteristics are listed in Table 1. There were no significant differences with respect to gender, age, pre-KPS, surgery type, location from the anal verge, clinical stage, pathologic stage, fractionation, and total dose between patients treated with IMRT versus 3DCRT. The mean number of elapsed treatment days was shorter for IMRT patients compared to 3DCRT patients (40.2 vs. 41.5 days, P=0.05) and patients receiving IMRT were more likely to receive concurrent capecitabine as opposed to infusional 5-FU (capecitabine 71.2% and 5-FU 17.3% vs. capecitabine 40.8% and 39.5%, P=0.03; respectively). IMRT patients were more likely to be treated supine (84.6%) than 3DCRT patients (14.5%; P=0.0001). Overall, a third of patients (n=31) received PET or MRI imaging and patients treated with IMRT were significantly more likely to receive PET or MRI imaging compared to 3DCRT (57% vs. 6%, P<0.001).
Full table
Acute toxicities
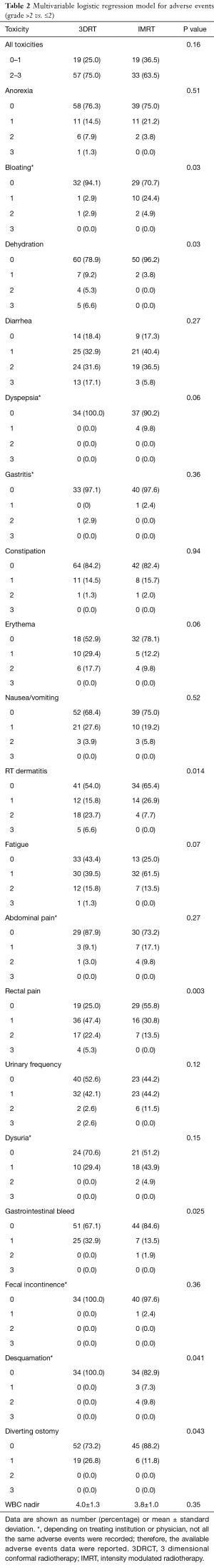
The use of IMRT was associated with an overall reduction in acute grade >2 gastrointestinal toxicities relative to 3DCRT (Table 2). Specifically, rates of grade >2 dehydration (0% vs. 6.6% P=0.03), dermatitis (0% vs. 6.6%, P=0.014) and proctitis (0% vs. 5.3%, P=0.003) were significantly lower in the IMRT cohort with a trend towards improved dyspepsia, pelvic skin erythema and fatigue (P=0.06, 0.06 and 0.07, respectively). Additionally, there was a decrease in the rate of diverting ostomies (11.8% vs. 26.8%, P=0.043) and grade 1 rectal bleeding (13.5% vs. 32.9%, P=0.025) with IMRT relative to 3DCRT. There was no difference in white blood cell count nadir during treatment with IMRT or 3DCRT (P=0.35).
Full table
PR rates
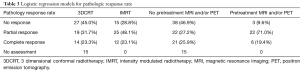
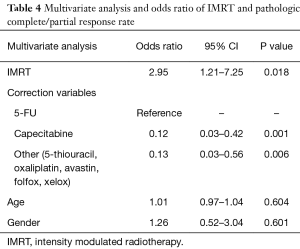
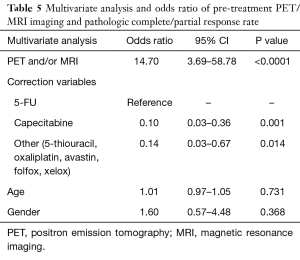
Definitive oncologic resection was performed for all 128 patients. Total mesorectal excision was performed on all patients with low anterior resection or abdominoperineal resection utilized in 61.4% and 38.6% of patients, respectively (Table 1). Pathologic data was available for 112 of 128 patients (n=52 IMRT and n=60 3DCRT patients). Of those receiving IMRT, 48.1% had a partial response, 23.1% had a complete response and 28.8% had no response (Table 3). Patients treated with 3DCRT had a partial response rate of 31.7%, a complete response rate of 23.3%, and no response in 45.0% (Table 3). Of those undergoing pre-treatment PET and/or MRI, 71.0% had a partial response, 19.4% had a complete response and 9.6% had no response (Table 3). Patients not undergoing pre-treatment PET and/or MRI had a partial response rate of 27.2%, a complete response rate of 25.9%, and no response in 46.9% (Table 3). After adjusting for age, gender, and pre-treatment chemotherapy, IMRT was significantly associated with increased odds for complete and partial response (OR =2.95; 95% CI: 1.21–7.25; P=0.018) (Table 4). Additionally, pre-treatment PET and/or MRI imaging was significantly associated with increased odds for complete and partial response (OR =14.70; 95% CI: 3.69–58.78; P<0.0001), after adjusting for age, gender, and pre-treatment chemotherapy (Table 5).
Full table
Full table
Full table
Discussion
Herein, we demonstrate a reduction in acute GI toxicities and improvement of PR rates with the use of IMRT compared to 3DCRT-based neoadjuvant chemoradiation (NeoCRT) for patients with LARC. Our outcomes are consistent with, and expand on previously published series (11,14-17). The largest and most recent of these studies by Ng et al. similarly identified an approximate 50% reduction of acute grade 2 and 3 diarrhea and genitourinary toxicities for patients receiving concurrent 5-FU-based chemotherapy and IMRT relative to 3DCRT. While our study did not identify reduced toxicities for the same adverse events, it adds to the body of literature suggesting IMRT offers clinically significant improved toxicity profile compared to 3DCRT.
In addition to reduced GI toxicities, our findings suggest use of IMRT and pre-treatment PET/MRI for delineation of radiotherapy treatment volumes resulted in improved PR rates. To our knowledge, this is the first study to show an improvement in PR, which is known to be prognostic of OS (18), and is often used as a surrogate for outcomes in LARC. In fact, degree of PR can influence adjuvant chemotherapeutic treatment options (19). For example, Collette et al. suggest that adjuvant chemotherapy only benefits patients who have had down staging of primary tumor from neoadjuvant therapy. The long term EORTC 22921 update also did not find any benefit to adjuvant chemotherapy regardless of down staging (20). This has prompted ongoing studies to further clarify the predictive significance of down staging and response to adjuvant chemotherapy (NCT01941979). The observed improvement in PR rates associated with IMRT and pre-treatment PET/MRI in this study is likely multifactorial.
Dosimetric studies comparing IMRT to 3DCRT in rectal cancer have shown superior target coverage, homogeneity, and conformality with IMRT (5). These dosimetric advantages allow lower doses to OAR and potential for higher doses to CTV, which can lead to improved PR rates. In fact, a recent meta-analysis has shown that doses above 60 Gy have been shown to improve PR rates (21). While we did not have doses above 60 Gy in our study, it is likely our patients treated with IMRT had improved target coverage allowing for a more complete and homogenous dose delivery (5). Accurate coverage of tumor volumes can be markedly improved with the use of PET and MRI (7,22). Prospective planning studies utilizing pre-treatment PET and MRI have led to changes in shape and size of GTVs in up to 72% of treatment plans and have identified new lesions in 15% of patients often leading to a change in treatment strategy (7,22).
Fewer treatment breaks and total elapsed days during neoadjuvant chemoradiotherapy have been shown to influence toxicity rates and clinical outcomes. Jabbour et al. have shown use of neoadjuvant IMRT for rectal cancer contributed to fewer hospitalizations, emergency department visits and ultimately unplanned treatment breaks (17). Additionally, Bitterman et al. found that unplanned treatment breaks during rectal cancer radiotherapy were associated with a greater risk of disease recurrence (23). Similarly, neoadjuvant radiotherapy delivered over a period exceeding 40 days had a borderline association with disease free survival (HR =4.45; P=0.052) (24). Whereas use of IMRT only trended to fewer treatment breaks (P=0.09) in our study, fewer total elapsed treatment days during NeoCRT was significant (P=0.05). Additionally, the use of image guided radiotherapy (IGRT) may have contributed to the improved outcomes seen with the use of IMRT in our study. Patients treated with IMRT often undergo daily IGRT with cone beam CT (CBCT) to ensure minimal anatomical variation thereby allowing smaller target volume margins. It has been shown that the CTV during radiotherapy treatment during the treatment of rectal cancer can have an average displacement of up to 0.7 mm – 1 cm depending on the anatomical axis (25). Similar results of rectal volume variation have been shown in the treatment of prostate and bladder cancers (26,27). These anatomical variations in set up, may produce geographic misses in patients treated with 3DCRT, as daily IGRT with CBCT was less likely to be used with 3DCRT treatment.
There are limitations associated with our study. As a multi-institutional study, differences in accepted treatment plans between treatment centers and pathologist determination of PR may confound our results. The study is retrospective and potentially biased by physician’s discretion as to who may benefit from IMRT vs. 3DCRT may have influenced outcomes. Furthermore, only pathologic down staging data, which is an established surrogate for clinical outcomes were collected. Our study results are consistent with and build upon a growing body of retrospective literature that supports the use of neoadjuvant chemotherapy-IMRT for LARC by virtue of reduced acute toxicities and improved PR rates relative to 3DCRT. Furthermore, pre-treatment PET and MRI imaging used almost exclusively with IMRT was associated with improved PR rates. In the absence of prospective data, PET/MRI-based-IMRT should be strongly considered for the treatment of LARC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by institutional review board of Cedars Sinai Medical Center (Pro00033479) and a waiver of informed consent was approved by the institutional review board.
References
- Sauer R, Liersch T, Merkel S, et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol 2012;30:1926-33. [Crossref] [PubMed]
- Guren MG, Dueland S, Skovlund E, et al. Quality of life during radiotherapy for rectal cancer. Eur J Cancer 2003;39:587-94. [Crossref] [PubMed]
- Bruheim K, Tveit KM, Skovlund E, et al. Sexual function in females after radiotherapy for rectal cancer. Acta Oncol 2010;49:826-32. [Crossref] [PubMed]
- Arbea L, Ramos LI, Martinez-Monge R, et al. Intensity-modulated radiation therapy (IMRT) vs. 3D conformal radiotherapy (3DCRT) in locally advanced rectal cancer (LARC): dosimetric comparison and clinical implications. Radiat Oncol 2010;5:17. [Crossref] [PubMed]
- Mok H, Crane CH, Palmer MB, et al. Intensity modulated radiation therapy (IMRT): differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol 2011;6:63. [Crossref] [PubMed]
- Bassi MC, Turri L, Sacchetti G, et al. FDG-PET/CT imaging for staging and target volume delineation in preoperative conformal radiotherapy of rectal cancer. Int J Radiat Oncol Biol Phys 2008;70:1423-6. [Crossref] [PubMed]
- Brændengen M, Hansson K, Radu C, et al. Delineation of gross tumor volume (GTV) for radiation treatment planning of locally advanced rectal cancer using information from MRI or FDG-PET/CT: a prospective study. Int J Radiat Oncol Biol Phys 2011;81:e439-45. [Crossref] [PubMed]
- Mitra D, Hong TS, Horick N, et al. Long-term outcomes and toxicities of a large cohort of anal cancer patients treated with dose-painted IMRT per RTOG 0529. Adv Radiat Oncol 2017;2:110-7. [Crossref] [PubMed]
- Klopp AH, Yeung AR, Deshmukh S, et al. A Phase III Randomized Trial Comparing Patient-Reported Toxicity and Quality of Life (QOL) During Pelvic Intensity Modulated Radiation Therapy as Compared to Conventional Radiation Therapy. Int J Radiat Oncol Biol Phys 2016;96:S3. [Crossref]
- Peng G, Wang T, Yang KY, et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy vs. conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother Oncol 2012;104:286-93. [Crossref] [PubMed]
- Hong TS, Moughan J, Garofalo MC, et al. NRG Oncology Radiation Therapy Oncology Group 0822: A Phase 2 Study of Preoperative Chemoradiation Therapy Using Intensity Modulated Radiation Therapy in Combination With Capecitabine and Oxaliplatin for Patients With Locally Advanced Rectal Cancer. Int J Radiat Oncol Biol Phys 2015;93:29-36. [Crossref] [PubMed]
- Wong SJ, Winter K, Meropol NJ, et al. Radiation Therapy Oncology Group 0247: a randomized Phase II study of neoadjuvant capecitabine and irinotecan or capecitabine and oxaliplatin with concurrent radiotherapy for patients with locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1367-75. [Crossref] [PubMed]
- Myerson RJ, Garofalo MC, El Naqa I, et al. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys 2009;74:824-30. [Crossref] [PubMed]
- Ng SY, Colborn KL, Cambridge L, et al. Acute toxicity with intensity modulated radiotherapy versus 3-dimensional conformal radiotherapy during preoperative chemoradiation for locally advanced rectal cancer. Radiother Oncol 2016;121:252-7. [Crossref] [PubMed]
- Samuelian JM, Callister MD, Ashman JB, et al. Reduced acute bowel toxicity in patients treated with intensity-modulated radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys 2012;82:1981-7. [Crossref] [PubMed]
- Parekh A, Truong MT, Pashtan I, et al. Acute gastrointestinal toxicity and tumor response with preoperative intensity modulated radiation therapy for rectal cancer. Gastrointest Cancer Res 2013;6:137-43. [PubMed]
- Jabbour SK, Patel S, Herman JM, et al. Intensity-modulated radiation therapy for rectal carcinoma can reduce treatment breaks and emergency department visits. Int J Surg Oncol 2012;2012. [Crossref] [PubMed]
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770-6. [Crossref] [PubMed]
- Collette L, Bosset JF, den Dulk M, et al. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol 2007;25:4379-86. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [Crossref] [PubMed]
- Burbach JP, den Harder AM, Intven M, et al. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol 2014;113:1-9. [Crossref] [PubMed]
- Wang YY, Zhe H. Clinical application of multimodality imaging in radiotherapy treatment planning for rectal cancer. Cancer Imaging 2013;13:495-501. [Crossref] [PubMed]
- Bitterman DS, Resende Salgado L, Moore HG, et al. Predictors of Complete Response and Disease Recurrence Following Chemoradiation for Rectal Cancer. Front Oncol 2015;5:286. [Crossref] [PubMed]
- Russo AL, Ryan DP, Borger DR, et al. Mutational and clinical predictors of pathologic complete response in the treatment of locally advanced rectal cancer. J Gastrointest Cancer 2014;45:34-9. [Crossref] [PubMed]
- Nuyttens JJ, Robertson JM, Yan D, et al. The variability of the clinical target volume for rectal cancer due to internal organ motion during adjuvant treatment. Int J Radiat Oncol Biol Phys 2002;53:497-503. [Crossref] [PubMed]
- Stasi M, Munoz F, Fiorino C, et al. Emptying the rectum before treatment delivery limits the variations of rectal dose - volume parameters during 3DCRT of prostate cancer. Radiother Oncol 2006;80:363-70. [Crossref] [PubMed]
- Lotz HT, Pos FJ, Hulshof MC, et al. Tumor motion and deformation during external radiotherapy of bladder cancer. Int J Radiat Oncol Biol Phys 2006;64:1551-8. [Crossref] [PubMed]


