Retrospective comparison of the efficacy and the toxicity of standard and modified FOLFIRINOX regimens in patients with metastatic pancreatic adenocarcinoma
Introduction
Metastatic pancreatic adenocarcinoma (MPA) represents a highly lethal condition. At the population level, a median overall survival of 2 months and a 5-year survival rate of 3.7% have been reported (1,2). Until recently, treatment efficacy fell short of expectations even in the setting of controlled randomized trials, and no single study had been able to demonstrate clinically meaningful and statistically significant benefits in overall survival of Gemcitabine-based combination chemotherapy over Gemcitabine alone (3). Thus, treatment improvements have been eagerly pursued in the management of MPA.
The randomized trial ACCORD 11/PRODIGE 4 stands as major breakthrough in the management of MPA. In this study, Conroy et al. were able to demonstrate benefits in median overall survival (11.1 vs. 6.8 months), median progression-free survival (6.4 vs. 3.3 months) and overall response rate (31.6% vs. 9.4%) with the use of FOLFIRINOX when compared to Gemcitabine (4). Quality of life analysis also favored treatment with FOLFIRINOX (5). Moreover, these results have been further reproduced in a similar phase 3 trial held in India (6). Perhaps more importantly, the feasibility of FOLFIRINOX outside the setting of a clinical trial has been demonstrated and the survival figures of the pivotal trial have also been reproduced in the clinical practice (7,8).
Unfortunately, the incremental activity of FOLFIRINOX comes at the expense of increased toxicity. High rates of grades 3–4 neutropenia (45.7%), fatigue (23.6%), vomiting (14.5%) and diarrhea (12.7%) have been reported in ACCORD 11/PRODIGE 4. Also, the low rate of febrile neutropenia (5.4%) reported in this trial has not been confirmed in some investigations, particularly in Asian patients and in the real-world setting (9-11). Indeed, in the later scenarios, rates of febrile neutropenia slightly above 20% have been reported and, as a result, FOLFIRINOX is frequently administered in conjunction with granulocyte colony-stimulating factor (G-CSF). Besides, FOLFIRINOX has been tested in clinical trials in rather selected populations, comprised of relatively young and fit patients. Thus, concerns regarding the toxicity profile of standard FOLFIRINOX prompted the evaluation of modified (attenuated) FOLFIRINOX regimens.
Some studies have addressed the activity and toxicity of modified FOLFIRINOX in MPA. As a general rule, similar survival outcomes to those described in the FOLFIRINOX arm of the ACCORD 11/PRODIGE 4 study have been shown (7,12-16). Additionally, a more tolerable side-effect profile has been demonstrated. Nevertheless, there is a shortage of comparative studies evaluating the outcomes of patients treated with standard FOLFIRINOX or with modified FOLFIRINOX (7) as most investigators describe the activity and the toxicity of their modified FOLFIRINOX regimens while comparing their results with those achieved in the FOLFIRINOX arm of the pivotal trial. Also, except for two Asian investigations, all previous studies have used mixed populations consisting of patients with locally advanced and metastatic pancreatic cancer (14).
Hence, we undertook a retrospective study to evaluate differences in outcomes of patients with MPA treated with standard or with modified FOLFIRINOX as first-line treatment. Additionally, we evaluated potential prognostic factors for patients with MPA treated with FOLFIRINOX in 1st line.
Methods
Design
This is a retrospective study, performed in a single cancer-dedicated hospital in Brazil. It was based on routinely collected data extracted from electronic charts of patients with MPA submitted to first-line treatment with FOLFIRINOX. Data were collected from June 2017 to December 2017. This study was approved by the AC Camargo Cancer Center Internal Ethics Board Review (CAAE 822894.5.0000.5432).
Patients
Inclusion criteria encompassed: age ≥18 years old, pathologically confirmed diagnosis of pancreatic adenocarcinoma, ECOG performance status 0–2, previously untreated metastatic disease and treatment with at least one cycle of FOLFIRINOX in first-line from January 1st 2010 through December 31th 2016. We excluded patients treated outside AC Camargo Cancer Center and those with concomitant metastatic malignancies. Patients submitted to at least one cycle of FOLFIRINOX in this setting were evaluated for efficacy and toxicity.
Treatment
Standard FOLFIRINOX consisted of Oxaliplatin 85 mg/m2 IV infused over 120 minutes, Irinotecan 180 mg/m2 IV infused over 90 minutes, Folinic Acid 400 mg/m2 IV infused over 30 minutes, 5-Fluorouracil 400 mg/m2 IV in bolus infusion and 5-Fluorouracil 2,400 mg/m2 IV in continuous infusion over 46 hours. Modified FOLFIRINOX consisted of Oxaliplatin 50–85 mg/m2, Irinotecan 60–180 mg/m2, Folinic Acid 0–400 mg/m2, bolus 5-Fluorouracil 0–400 mg/m2 and continuous infusion 5-Fluorouracil 1,800–2,400 mg/m2. The same infusion protocols were used for standard and modified FOLFIRINOX and in both FOLFIRINOX protocols, cycles were repeated every 14 days. Patients who underwent at least one cycle of full-dose FOLFIRINOX constitute the standard FOLFIRINOX group. The remaining patients constitute the modified FOLFIRINOX group.
Patients routinely received Dexamethasone 10 mg IV and Ondansetron 12 mg IV immediately before the start of each cycle as chemotherapy-induced nausea and vomiting prophylaxis. Patients were oriented to use Ondansetron 8 mg PO every 8 hours afterwards up to the third day of chemotherapy. According to the tolerance to chemotherapy, further escalation of anti-emetics was implemented. Atropine (0.5 mg) was administered before the infusion of Irinotecan in order to avoid acute cholinergic symptoms. In some patients, primary prophylaxis with G-CSF was performed at the discretion of the treating physician. This was accomplished by the use of Filgrastrim 300 mcg SC for 2 to 6 days, or by a single subcutaneous injection of either pegfilgrastim or lipegfilgrastim (both 6 mg). G-CSF was started on the fourth day of the chemotherapy cycle.
Procedures
Data regarding response were extracted from original radiological reports and there was no independent radiological imaging review. Assessment of tumor response was performed every four to six cycles with multi-detector computer tomography (MD-CT) or magnetic resonance imaging (MRI) or both. Biochemical tumor response was described according to changes in tumor markers after the beginning of treatment. Such measurements took place every four to six cycles. Patients with baseline serum CA 19-9 ≤37 U/mL or baseline serum CEA ≤5 ng/mL were considered to have a normal tumor marker level. Baseline tumor markers were collected at the diagnosis of metastatic disease, regardless of the biliary tract patency.
Predictor variables
We collected data on baseline patients’ characteristics: age, gender, ECOG performance status, Age-Adjusted Charlson Comorbidity Score (AACCS), body mass index (BMI), tumor site (head/neck vs. body/tail), number of metastatic sites, serum CA 19-9 at diagnosis of metastatic disease (U/mL), serum CEA at diagnosis of metastatic disease (ng/mL) and neutrophil-to-lymphocyte ratio (NLR) at diagnosis of metastatic disease. Patients for whom performance status was not available had their performance inferred from the description of patients’ capabilities found in the medical records.
We also gathered information on the delivered treatment: dose (in mg/m2) of each drug in the first FOLFIRINOX cycle, number of cycles of FOLFIRINOX (total, before treatment de-escalation and after treatment de-escalation) and primary use of G-CSF. Additionally, we gleaned data on further lines of treatment. We assessed: the number of lines of treatment, percentage of patients treated in second- and third-line settings and the types of chemotherapy used in second- and third-line treatment.
Outcomes
The primary outcome of the study was overall survival (OS) according to treatment with either standard FOLFIRINOX or modified FOLFIRINOX. As secondary outcomes, we assessed differences in progression-free survival (PFS), biochemical response rates (for CA 19-9 and CEA), radiological tumor response rate and toxicity between these two groups. OS was defined as the time from the start of FOLFIRINOX to death (from any cause). Progression-free survival (PFS) was defined as the time from the start of FOLFIRINOX to death or disease progression (whatever took place first). Patients were censored at last follow-up visit in the absence of an event. We defined biochemical response as at least a 50% reduction in tumor marker level from baseline. Patients with normal levels of serum tumor markers at diagnosis were considered non-assessable for these analyses. Radiological tumor response was assessed using RECIST 1.1 criteria. Toxicity was graded according to the Common Toxicity Criteria version 4.0. Safety profile also included analysis of treatment delays (any delay), treatment de-escalations (withdrawal of either Oxaliplatin or Irinotecan or both), dose reductions (any dose reduction or withdrawal of bolus 5-Fluorouracil before treatment de-escalation), treatment-related mortality and severe toxicity (treatment-related complication mandating hospital admission). Toxicity was assessed not only in the first, but in all cycles of FOLFIRINOX.
Statistical analysis
We used absolute values and ratios to describe the distribution of categorical variables. Distributions of categorical variables were compared using Fisher’s exact test. We used median values and the interquartile range (IQR) to describe the distribution of numerical variables. For chemotherapy dose at first cycle of FOLFIRINOX, we also used mean and standard deviation values. Distributions of numerical variables were compared using the Mann-Whitney test. We generated curves to describe time-to-event variables (OS and PFS) according to the Kaplan-Meier method. Survival curves were compared using the log-rank test. We used Cox’s Proportional Hazard method to performed univariate analysis on OS and PFS. Variables with Wald’s P value <0.20 in univariate analysis were selected for multivariate analysis. As baseline NLR, serum CA 19-9 and serum CEA showed extremely right-skewed distributions, for didactic purposes, we transformed these variables to the logarithmic base (log10) in the regression model. We considered two-tailed P values <0.05 as statistically significant. For the numerical variables independently associated with OS, we performed cut-off analysis based on the maximally selected rank statistics method (17). Statistical analysis was performed with the software R Project version 3.4.0 along with the survival and MaxStat packages.
Results
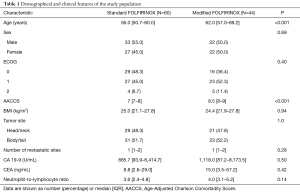
Through a search in the AC Camargo Cancer Center Pancreatic Cancer Database, we identified 118 patients treated for MPA with first-line FOLFIRINOX from January 1st 2010 through December 31th 2016 (Figure 1). Fourteen patients were excluded due to: ECOG 3 (2 patients), concomitant metastatic malignancy (1 patient) and treatment performed outside AC Camargo Cancer Center (11 patients). Table 1 describes the characteristics of the study population. The median age was 59 years old (IQR, 52.7–65.0 years old). Most patients were male (52.9%, N=55). Only 8.7% of patients presented ECOG 2 and the median AACCS was 8 (IQR, 7–9). Most patients were eutrophic according to BMI status (median BMI =24.5; IQR, 21.3–27.8). Nearly half of the primary tumors were located in the pancreatic head/neck (48.1%, N=50) and most patients presented a single site of metastasis (median number of metastatic sites =1; IQR, 1–2). Median serum CA 19-9 at diagnosis was 1,114.7 U/mL (IQR, 90.3–6,549.0 U/mL), median serum CEA at diagnosis was 10.1 ng/mL (IQR, 2.9–47.4 ng/mL) and median NLR at diagnosis was 3.7 (IQR, 3.0–5.1). Comparing both treatment groups, patients treated with standard FOLFIRINOX were younger (P<0.001) and presented lower AACCS (P<0.001).
Full table
Treatment
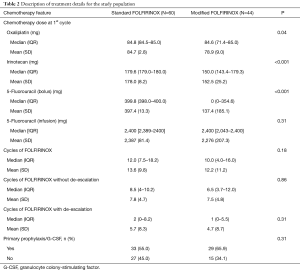
Table 2 depicts treatment characteristics in the two groups. The standard FOLFIRINOX arm comprise 60 patients and 44 patients constitute the modified FOLFIRINOX arm. Two patients that initiated treatment with modified FOLFIRINOX and after one cycle had treatment escalation to full-dose FOLFIRINOX were included in the standard FOLFIRINOX group. During the first cycle of FOLFIRINOX, similar doses of continuous infusion 5-Fluorouracil were administered in both groups. Conversely, lower doses of Oxaliplatin, Irinotecan and bolus 5-Fluorouracil were given in the modified FOLFIRINOX group. Also, there was no difference in the total number cycles of FOLFIRINOX delivered, as well as in the number of FOLFIRINOX cycles before or after treatment de-escalation.
Full table
Efficacy
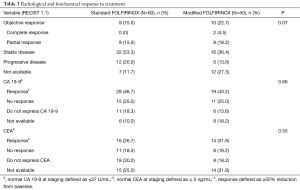
Table 3 illustrates the radiological and biochemical responses to treatment. There were no differences in serum CEA and serum CA 19-9 changes in response to treatment between the two treatment arms. The objective response rate was numerically higher in patients treated with modified FOLFIRINOX (standard FOLFIRINOX =15.0% vs. modified FOLFIRINOX =22.7%), but this difference was not statistically significant (P=0.07). After excluding patients not expressing a specific tumor marker and those not assessed for biochemical response, statistically significant associations were seen between CEA response (N=40; Fisher exact test P=0.03) and radiological response to chemotherapy and between CA 19-9 response (N=65; Fisher exact test P=0.001) and radiological response to chemotherapy.
Full table
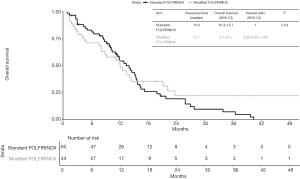
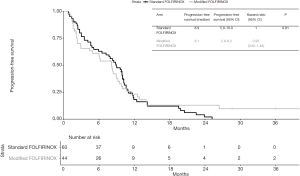
Median follow-up time was 28.6 months (95% CI 20.8–NA). At last follow-up, 49 patients in the standard FOLFIRINOX arm were dead and 27 patients in the modified FOLFIRINOX arm were dead. No difference in OS was detected between the two treatment groups. Median overall survival was 13.4 months in the standard FOLFIRINOX arm and 12.1 months in modified FOLFIRINOX arm (HR =0.86; 95% CI, 0.53–1.38; P=0.54; Figure 2). One- and two-year OS rates were 56.4% and 19.3% in the standard FOLFIRINOX group and 54.8% and 22.2% in the modified FOLFIRINOX group, respectively. In the multivariate model, no difference in overall survival was detected between the two treatment arms (HR =0.94; 95% CI, 0.51–1.72; P=0.84; Table 4). At last follow-up, 56 patients were dead or experienced disease progression in the standard FOLFIRINOX arm and 38 patients were dead or experienced disease progression in the modified FOLFIRINOX arm. No difference in progression-free survival was detected between the two treatment arms. Median progression-free survival was 8.9 months in the standard FOLFIRINOX arm and 8.1 months in the modified FOLFIRINOX arm (HR =0.95; 95% CI, 0.62–1.44; P=0.81; Figure 3). One- and 2-year PFS rates were 18.0% and 2.0% in the standard FOLFIRINOX group and 23.1% and 12.8% in the modified FOLFIRINOX group, respectively. In the multivariate model, no difference in progression-free survival was detected between the two treatment arms (HR =1.13; 95% CI, 0.65–1.96; P=0.66; Table 5).
Full table
Full table
Prognostic factors
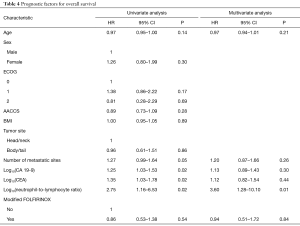
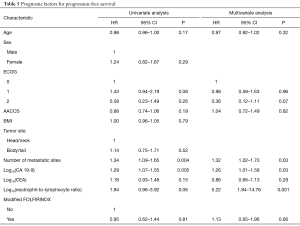
Tables 4,5 describe the prognostic factors for OS and PFS, respectively. In the multivariate analysis, only log10(NLR) was independently associated with OS (HR =3.60; 95% CI, 1.28–10.10; P=0.01). In turn, the number of metastatic sites (HR =1.32; 95% CI, 1.02–1.70; P=0.03), log10(CA 19-9) (HR =1.26; 95% CI, 1.01–1.58; P=0.03) and log10(NLR) (HR =5.22; 95% CI, 1.84–14.76; P=0.001) were independent predictors of PFS. Cut-off analysis revealed 5.2 represented the best NLR threshold for OS. After dichotomizing neutrophil/lymphocyte ratio (≤5.2 vs. >5.2), significant differences in OS (HR =2.74; 95% CI, 1.54–4.87; P<0.001) and PFS (HR =2.58; 95% CI, 1.52–4.37; P<0.001) were observed. Also, after excluding patients not assessable for tumor response, response to treatment according to RECIST (complete or partial response) was related to better OS (HR =0.42; 95% CI, 0.22–0.79; P=0.007) and PFS (HR =0.44; 95% CI, 0.25–0.76; P=0.003) in comparison to patients experiencing stable or progressive disease.
Toxicity
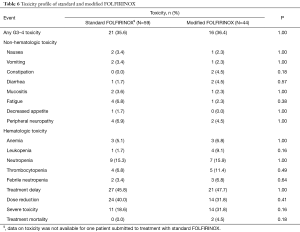
Table 6 illustrates the toxicity profiles of standard and modified FOLFIRINOX. Overall, there were no major differences in toxicity between the two treatment arms. Slightly higher rates of grades 3 or 4 constipation (0% vs. 4.5%) and leukopenia (1.7% vs. 9.1%) were seen in patients in the modified FOLFIRINOX group. In turn, mildly increased incidence of fatigue was seen in patients treated with standard FOLFIRINOX (6.8% vs. 2.3%). Also, chemotherapy dose reductions were more common in the standard FOLFIRINOX arm (40.0% vs. 31.8%). Conversely, toxicities mandating hospitalization (18.6% vs. 31.8%) and treatment-related mortality (0.0% vs. 4.5%) were more frequent in the modified FOLFIRINOX arm. None of these differences were statistically significant. Two patients died as a consequence of the treatment. In the modified FOLFIRINOX arm, one patient died as a consequence of sinonasal mucormycosis and another patient died of febrile neutropenia/intraabdominal infection.
Full table
Further treatment
Table 7 describes therapy beyond first-line in both treatment arms. Patients in the standard FOLFIRINOX arm were more prone to receive second- (P=0.007) and third-line treatments (P=0.06). Overall, the median number of treatment lines also was higher in the standard FOLFIRINOX arm (P=0.002). Gemcitabine based-chemotherapy regimens were among the most commonly used therapies both in second- and in third-line.
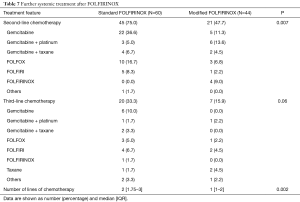
Full table
Discussion
Data from randomized clinical trials have established FOLFIRINOX as one of the standard treatment regimens for patients with MPA. Nonetheless, it has been primarily tested in patients relatively young and with good performance status. Even in this selected group, high rates of toxicity, namely neutropenia, have been described. As a consequence, modifications of this regimen have been tested to improve the toxicity profile of FOLFIRINOX. In this study, we detected no differences in PFS or OS between patients treated with standard FOLFIRINOX or with modified FOLFIRINOX. Also, we demonstrated that reductions in the FOLFIRINOX dose had no impact in the response according to tumor marker kinetics or RECIST.
Our survival data are in line with other investigations evaluating the role of modified FOLFIRINOX in patients with MPA. The first prospective trial of modified FOLFIRINOX in MPA was published by Stein et al. (12). They enrolled 31 patients with locally advanced pancreatic adenocarcinoma and 37 patients with MPA to treatment with attenuated doses of FOLFIRINOX (Irinotecan 135 mg/m2 and bolus 5-Fluorouracil 300 mg/m2). The response rate was 35.1% in the cohort of patients with metastatic disease. Median PFS and median OS in this group were 6.1 and 10.2 months, respectively. Ueno et al. undertook a phase 2 study evaluating the activity of a modified FOLFIRINOX regimen (Irinotecan 150 mg/m2 and no 5-Fluorouracil bolus) in 69 patients with metastatic pancreatic cancer (13). The objective response rate was 37.7%. Median PFS and median OS were 5.5 and 11.2 months, respectively. Similarly, Li et al. conducted a phase 2 trial exclusively in patients with MPA testing a modified FOLFIRINOX regimen with reduced doses of both Oxaliplatin (85% of standard dose) and Irinotecan (75% of standard dose), along with the omission of bolus 5-Fluorouracil (14). Among the 62 eligible patients, the overall response rate was 32.5%. Median PFS and median OS were 7.0 and 10.3 months, respectively. These outcomes are similar to the ones found in the ACCORD 11/PRODIGE 4 study. In this study, the overall response rate was 31.6%; median PFS and median OS were 6.8 and 11.1 months, respectively. Additionally, a retrospective Canadian study have shown similar survival for patients with MPA treated with standard or modified FOLFIRINOX regimens (7). Taken together, this data point to a similar activity of modified FOLFIRINOX as compared do standard FOLFIRINOX.
Despite the evidence in favor of modified FOLFIRINOX, the proper doses of the different agents in this regimen are still a source of argument, as a variety of doses have been used. Recent data point the doses of the different drugs used in FOLFIRINOX must be significantly reduced before a loss of effectiveness is observed. Decreased response rate and disease control rate have been show to occur only after the median dose intensity of FOLFIRINOX is reduced to less than 70% and 55%, respectively (18). In our study, dose reductions were left to discretion of the treating physician. As a result, different modified FOLFIRINOX regimens were used. In most cases, the doses of Oxaliplatin and continuous infusion 5-Fluorouracil were maintained. The relevant differences lied on the doses of bolus 5-Fluorouracil and Irinotecan. Most of the patients treated with modified FOLFIRINOX had bolus 5-Fluorouracil omitted. This is similar to what has been reported in prospective (13,14) and retrospective studies of modified FOLFIRINOX in pancreatic cancer (16,19,20). In colorectal cancer, the omission of bolus 5-Fluorouracil has shown to improve the toxicity profile while maintaining treatment activity (21,22). Even in anatomical borderline pancreatic adenocarcinoma, a setting in which optimization of response is pursued in order to achieve R0 resections, FOLFIRINOX has been recently employed without bolus 5-Fluorouracil (23). Thus, these arguments speak in favor of omitting bolus 5-Fluorouracil from FOLFIRINOX in patients with MPA.
In our study, the mean dose and the median dose of Irinotecan in the first cycle of modified FOLFIRINOX were 152.5 and 150 mg/m2, respectively. In previous investigations, modified FOLFIRINOX regimens usually had decreased doses of Irinotecan, ranging from 135 to 165 mg/m2 (12-14,16,19,24). Irinotecan adds significantly to the occurrence of nausea, vomiting and diarrhea when used in triplet-chemotherapy regimens. In patients treated with FOLFOXIRI, a regimen with a decrease in the dose of Irinotecan to 165 mg/m2, the frequency of grade 3–4 vomiting (3.6%) is significantly lower than that found in the ACCORD 11/PRODIGE 4 trial (14.5%) with standard FOLFIRINOX (25). Also, reduced activity of UGT1A have been detected in a significant proportion of the population and individuals carrying this enzymatic deficiency are at increased risk for severe toxicity when treated with Irinotecan (26,27). In Brazil, routine testing for UGT1A deficiency is not available before starting Irinotecan, and we believe by starting FOLFIRINOX with decreased doses of Irinotecan in these patients we may lower the risk of severe toxicities (namely neutropenia and diarrhea) that occur in the first few cycles of treatment (9). As a result, dose reductions in Irinotecan can be employed in the first cycle and we consider 150 mg/m2 a reasonable dosing choice in this setting.
The overall response rate observed in our study is somewhat lower than previously described (4,6,12). That may be explained by the lack of independent radiological review. Also, some studies describe response rates only for the assessable patients (7,28). Had we done that, our response rate would be 31.2% and 16.9% for modified and standard FOLFIRINOX, respectively. These figures approach the response rate observed in clinical trials using FOLFIRINOX in MPA (4,6,12). However, we believe that many patients not assessable for tumor response experience early treatment failure or significant treatment toxicity, demonstrating a lack of treatment benefit. Despite the lower response seen in our study, there was a strong correlation between response according to RECIST and response according to CA 19-9 or CEA kinetics. Additionally, patients experiencing objective response to FOLFIRINOX showed markedly improved PFS and OS.
One of the main arguments in favor of a modification of FOLFIRINOX is a potential decrease in treatment toxicity. In our study, we were not able to demonstrate significant improvements in the toxicity profile with modified FOLFIRINOX when compared to standard FOLFIRINOX. Severe toxicities were relatively uncommon in our population. We acknowledge this may stem from the retrospective nature of our study. In addition, patients treated with modified FOLFIRINOX were less fit than those treated with standard FOLFIRINOX. They were older and presented more comorbidities according to the Age-Adjusted Charlson Comorbidity Score (AACCS). Moreover, they we also less likely to undergo further systemic treatments after progression on FOLFIRINOX. Thus, we believe the use of decreased doses of chemotherapy might have minimized the risk of complications in this group of patients. Also, the use of prophylactic G-CSF may have biased the toxicity analysis. Most of our patients had primary febrile neutropenia prophylaxis. The rates of grade 3-4 neutropenia in both groups were much lower than described in the ACCORD 11/PRODIGE 4 trial. Had that not been the case, we believe the rates of severe toxicity in the standard FOLFIRINOX would have been higher, since complications such as febrile neutropenia commonly occur in the first few cycles during treatment with FOLFIRINOX. As a result, further studies must be undertaken to evaluate the role of prophylactic G-CSF in lowering hematological toxicity during treatment with FOLFIRINOX.
In our study, NLR proved to be the most important predictor of outcome. Moreover, patients with NLR >5.2 presented the highest risk for early death. That is not specific for FOLFIRINOX, as similar data have also been reported for Gemcitabine + Nab-paclitaxel (29,30) and FOLFOXIRI (25). Also, the threshold for NLR found in our study matches the ones found in the literature (31). It is not clear from recent data if the NLR is only a marker of systemic inflammation or if the increased neutrophils have an independent biological meaning. Recent data point neutrophils may facilitate the occurrence of metastatic disease by means of direct interactions with circulating pancreatic cancer cells (32). Nonetheless, increased numbers of peripheral neutrophils may simply reflect higher concentrations of inflammatory cytokines, such as G-CSF. Recently, it has been demonstrated in a murine model that elevated tumor levels of G-CSF correlate with an aggressive tumor phenotype (33). This phenotype seems to be primarily driven by the immune-suppressive properties of G-CSF, and could be abrogated by treatment with an antibody directed against G-CSF. As a result, we believe additional work is needed to better understand the relation between NLR and the cytokine milieu in patients with MPA.
Our study presents some limitations. We acknowledge that as a single center retrospective study, bias may have played a role in the results. Also, we were not able to obtain independent radiology imaging review and serum CA 19-9 levels were analyzed in the presence of cholestasis in some patients. We also could not collect data on dose intensity beyond the first cycle of FOLFIRINOX. Nevertheless, we believe that the choice of the dose in the first cycle carries more practical implications than overall treatment dose intensity, as dose reductions are dictated not by intended dose-intensity, but by the presenting toxicity. Also, some of the most serious adverse events from FOLFIRINOX treatment frequently take place after the first cycle (9), emphasizing the need for careful dose selection for the first cycle. Moreover, this is one of the largest studies evaluating the role of modified FOLFIRINOX in pancreatic cancer. We analyzed its activity in a population comprised exclusively of patients with metastatic disease and treated in the same hospital, with access to similar support and medical expertise. Our survival results match those of studies previously published. In addition, we found significant associations between NLR and outcomes, with a threshold similar to those already described.
To conclude, modified FOLFIRINOX demonstrated similar activity to that of standard FOLFIRINOX in patients with MPA. No major differences in toxicity were observed, mainly due to selection bias, uneven dose reductions and unrestricted use of prophylactic G-CSF. Prognosis for patients with an elevated NLR is poor, and efforts should be concerted to better comprehend the biological mechanisms behind this phenomenon in order to improve treatment outcomes.
Acknowledgements
Thanks are due to Dr. Rachel Simões Pimenta Riechelmann for kindly proofreading the manuscript and advising on certain methodological issues.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the AC Camargo Cancer Center Internal Ethics Board Review (CAAE 822894.5.0000.5432). The study was IRB approved for waiver of informed consent as it’s a retrospective study.
References
- Golan T, Sela T, Margalit O, et al. Short and long-term survival in metastatic pancreatic adenocarcinoma, 1993-2013. J Natl Compr Canc Netw 2017;15:1022-7. [Crossref] [PubMed]
- Sirri E, Castro FA, Kieschke J, et al. Recent Trends in Survival of Patients with Pancreatic Cancer in Germany and the United States. Pancreas 2016;45:908-14. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med 2011;364:1817-25. [Crossref] [PubMed]
- Gourgou-Bourgade S, Bascoul-Mollevi C, Desseigne F, et al. Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. J Clin Oncol 2013;31:23-9. [Crossref] [PubMed]
- Singhal M, Kapoor A, Bagri P. A phase III trial comparing FOLFIRINOX versus Gemcitabine for metastatic pancreatic cancer. Ann Oncol 2014;25:iv210-53. [Crossref]
- Chllamma MK, Cook N, Dhani NC, et al. FOLFIRINOX for advanced pancreatic cancer: The Princess Margaret Cancer Centre experience. Br J Cancer 2016;115:649-54. [Crossref] [PubMed]
- Metges J, Ramée J, Douillard JY, et al. Efficacy and safety of FOLFIRINOX in patients with metastatic pancreatic cancer. J Clin Oncol 2014;32:abstr 305.
- Okusaka T, Ikeda M, Fukutomi A, et al. Phase II study of FOLFIRINOX for chemotherapy-naïve Japanese patients with metastatic pancreatic cancer. Cancer Sci 2014;105:1321-6. [Crossref] [PubMed]
- Weycker D, Li X, Edelsberg J, et al. Risk of febrile neutropenia in patients receiving emerging chemotherapy regimens. Support Care Cancer 2014;22:3275-85. [Crossref] [PubMed]
- Patel L, Hollmann S, Attard C, et al. Real-world experience with FOLFIRINOX: A review of Canadian and international registries. Oncol Exch 2014;13:18-23.
- Stein SM, James ES, Deng Y, et al. Final analysis of a phase II study of modified FOLFIRINOX in locally advanced and metastatic pancreatic cancer. Br J Cancer 2016;114:737-43. [Crossref] [PubMed]
- Ueno M, Ozaka M, Ishii H, et al. Phase II study of modified FOLFIRINOX for chemotherapy-naïve patients with metastatic pancreatic cancer. J Clin Oncol 2016;34:abstr 4111.
- Li X, Ma T, Zhang Q, et al. Modified-FOLFIRINOX in metastatic pancreatic cancer: A prospective study in Chinese population. Cancer Lett 2017;406:22-6. [Crossref] [PubMed]
- Gunturu K, Thumar J, Hochster H, et. Single-institution experience with FOLFIRINOX in advanced pancreatic cancer (PC). J Clin Oncol 2012;30:abstr 330.
- Mahaseth H, Brutcher E, Kauh J, et al. Modified FOLFIRINOX regimen with improved safety and maintained efficacy in pancreatic adenocarcinoma. Pancreas 2013;42:1311-5. [Crossref] [PubMed]
- Lausen B, Schumacher M. Maximally Selected Rank Statistics. Biometrics 1992;48:73-85. [Crossref]
- Lee JC, Kim JW, Ahn S, et al. Optimal dose reduction of FOLFIRINOX for preserving tumour response in advanced pancreatic cancer: Using cumulative relative dose intensity. Eur J Cancer 2017;76:125-33. [Crossref] [PubMed]
- Blazer M, Wu CS, Goldberg R, et al. Tolerability and efficacy of modified FOLFIRINOX (mFOLFIRINOX) in patients with borderline-resectable pancreatic cancer (BRPC) and locally advanced unresectable pancreatic cancer (LAURPC). J Clin Oncol 2014;32:abstr 275.
- Alessandretti M, Moreira R, Brandao E, et al. Safety and efficacy of modified dose- attenuated FOLFIRINOX chemotherapy in patients over 65 years with advanced pancreatic adenocarcinoma. J Clin Oncol 2015;33:abstr 468.
- Denda T, Kanda M, Morita Y, et al. Pharmacokinetic dose adjustment of 5-FU in modified FOLFOX7 plus bevacizumab for metastatic colorectal cancer in Japanese patients: a-JUST phase II clinical trial. Cancer Chemother Pharmacol 2016;78:1253-61. [Crossref] [PubMed]
- Tezuka T, Hamada C, Ishida H, et al. Phase II clinical study of modified FOLFOX7 (intermittent oxaliplatin administration) plus bevacizumab in patients with unresectable metastatic colorectal cancer—CRAFT study. Invest New Drugs 2013;31:1321-9. [Crossref] [PubMed]
- Katz MH, Shi Q, Ahmad SA, et al. Preoperative Modified FOLFIRINOX Treatment Followed by Capecitabine-Based Chemoradiation for Borderline Resectable Pancreatic Cancer: Alliance for Clinical Trials in Oncology Trial A021101. JAMA Surg 2016;151. [Crossref] [PubMed]
- Ghorani E, Wong HH, Hewitt C, et al. Safety and Efficacy of Modified FOLFIRINOX for Advanced Pancreatic Adenocarcinoma: A UK Single-Centre Experience. Oncology 2015;89:281-7. [Crossref] [PubMed]
- Vivaldi C, Caparello C, Musettini G, et al. First-line treatment with FOLFOXIRI for advanced pancreatic cancer in clinical practice: Patients’ outcome and analysis of prognostic factors. Int J Cancer 2016;139:938-45. [Crossref] [PubMed]
- Stingl JC, Bartels H, Viviani R, et al. Relevance of UDP-glucuronosyltransferase polymorphisms for drug dosing: A quantitative systematic review. Pharmacol Ther 2014;141:92-116. [Crossref] [PubMed]
- Rouits E, Boisdron-Celle M, Dumont A, et al. Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: A molecular and clinical study of 75 patients. Clin Cancer Res 2004;10:5151-9. [Crossref] [PubMed]
- Peddi PF, Lubner S, McWilliams R, et al. Multi-Institutional Experience with FOLFIRINOX in Pancreatic Adenocarcinoma. JOP 2012;13:497-501. [PubMed]
- Goldstein D, El-Maraghi RH, Hammel P, et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic cancer: Long-term survival from a phase III trial. J Natl Cancer Inst 2015;107:1-10. [Crossref] [PubMed]
- Ventriglia J, Alvaro M, Laterza M, et al. Final results of neutrophil-to-lymphocyte ratio (NLR) as a prognostic marker in advanced pancreatic cancer patients treated with Nab-paclitaxel plus Gemcitabine. J Clin Oncol 2016;34:abstr e15737.
- Piciucchi M, Stigliano S, Archibugi L, et al. The neutrophil/lymphocyte ratio at diagnosis is significantly associated with survival in metastatic pancreatic cancer patients. Int J Mol Sci 2017;18:3-11. [Crossref] [PubMed]
- Tao L, Zhang L, Peng Y, et al. Neutrophils assist the metastasis of circulating tumor cells in pancreatic ductal adenocarcinoma. Medicine (Baltimore) 2016;95. [Crossref] [PubMed]
- Pickup MW, Owens P, Gorska AE, et al. Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte-colony stimulating factor secretion in carcinoma cells. Cancer Immunol Res 2017;5:718-29. [Crossref] [PubMed]



