Demographic characteristics and the first program of colorectal cancer (CRC) screening in north of Iran (2016)
Introduction
Cancer is one of the leading causes of death in the world (1). It is predicted that cancer will be the first and most important cause of death in 2030 (2). Colorectal cancer (CRC) is one of the most common types of cancers in the gastrointestinal tract (3). It is also the third most common cancer in men (746,000 cases, 10% of all cancers) and the second most common cancer in women (614,000 cases, 9.2% of all cancers) worldwide (4). According to recent studies in northern part of Iran, CRC is one of the most common cancers and its trend has been increasing in recent years (5).
CRC screening using screening tests significantly increases the survival rate of cancer. This is why CRC screening is strongly encouraged in different countries (6). Ideally, a screening method should be a simple and inexpensive test to apply easily for the entire population at risk. This criterion does not meet the colonoscopy because it is a golden standard approach. So those individuals with an IFOBT-positive test should do colonoscopy procedure. There is compelling evidence in terms of cost-effectiveness of CRC screening for population at risk. In addition, screening is recommended for asymptomatic individuals too, because there are symptoms of CRC or polyps that require colonoscopy diagnostic examination (7). Clinical trials and systematic reviews have shown that the 2-year screening program, by performing IFOBT and follow up with colonoscopy, can reduce the damages induced by the CRC up to 16% (8,9). Also, colonoscopy permits diagnosis and removal of polyps and cancer biopsy throughout the colon. Besides, both the sensitivity and the specificity of colonoscopy are appropriate for the diagnosis of polyps and cancer (7).
The screening program depends on the level of people’s knowledge, as well as their willingness and motivation to participate in the program. So, the program should be carefully planned (10,11) and regarded by researchers and managers of the health sector. For a successful screening program, the following multiple factors should occur: starting with the doctor’s advice, patient hospitalization, financial support, risk classification, screening test, timely diagnosis, timely treatment, and appropriate follow-up; if any of these steps fails or has low quality, the screening cannot succeed (12).
Demographic characteristics should be considered for a successful screening program. The various determinants of CRC screening prognosis are stated in different studies including age, gender, marital status, higher education, place of residence, race or ethnicity, family history of CRC, and doctor’s recommendation (13-16). Among them, age is an important factor in screening results. CRC risk increases by age and family history. CRC is rare before age 50, but then the cancer risk increases significantly (12).
CRC is one of the most preventable cancers and there is no plan for its screening for the general population at medium risk in Iran. The current screening program is a colonoscopy for the first screening test and is only performed for high-risk individuals (first-degree relatives of the affected patients). The aim of this study was to examine the effect of demographic characteristics on all stages and its impact on the results of the first CRC screening program, including the results of IFOBT, as the most effective screening method, and the results of colonoscopy in people over 50 years old in northern part of Iran. This study also aimed to identify the variables of prognosis of the disease to help the community to prevent and control this kind of cancer.
Methods
This cross-sectional study was conducted from February to July 2016. Considering the high prevalence of positive FOBT based on the experts’ opinions and due to the potential drop of the participants during the study, the nature of screening and available resources, ultimately, the sample size included 924 individuals over 50 years old in Babol. At the beginning of the program, after coordination with the health department of Babol University of Medical Sciences, ten qualified health centers with full-time laboratories and full-time staff were selected as host centers. Regarding the population covered by each host center and considering the study as the first screening program for the medium risk individuals in the general population, sampling was performed using the convenience sampling method.
Exclusion and inclusion criteria were determined based on the study objectives. Inclusion criteria were individuals older than 50 years old, who were convinced to do the test in three stages and consented to cooperate till the end of the process. Exclusion criteria were the history of gastrointestinal surgery, diagnosed gastrointestinal diseases such as gastrointestinal ulcers, clear blood transfusions from the anus or the presence of visible blood in the feces, sinusitis, and blood in the sputum and secretions; For women, the days about to the menstrual period and up to 3 days after the end of the menstruation, bleeding from the gums and oral mucosa during brushing, dental floss or any other agent (in this case, the test was postponed for several days and the person was advised to avoid tooth brushing and dental flossing in these days) and failure to provide two acceptable test samples.
All of the personnel and heads of the host centers had received two sessions of training course about the program at the center for Colorectal Cancer Counseling and Screening. Also, all health staff of the centers provided notification about the referral of the target group and free of charge services. After referring the individuals to the health centers, they were provided with the explanation of the study objectives by trained interviewers. Then the questionnaires were completed through face-to-face interviews for those who signed the written informed consent. The study was approved by ethics committee of Shiraz University of Medical Sciences No. IR.SUMS.REC.1396.S5807. The questionnaire was prepared by the researchers, consisting of demographic characteristics: age, sex, marital status, education, height and weight (BMI), place of residence (city or village) and results of the tests and the colonoscopy. At the end of the interview, the necessary education and counseling were also provided to the subjects about the importance of screening, healthy lifestyle and convincing them for cooperation during the screening program. Then the participants were introduced to the laboratory for the three tests. The immunochemical method, called Immunochemical Fecal Occult Blood Test (IFOBT), was also used in this study.
Those participants for which the test result was positive (at least one positive result out of the three) were introduced to a state hospital (referring to the gastroenterologist at the colonoscopy unit of Shahid Beheshti and Ayatollah Rouhani Hospitals of Babol) for the colonoscopy to perform the necessary diagnostic and therapeutic procedures.
Regarding the study objectives and type of variables, SPSS 22 software was used to analyze the data. Descriptive statistics (frequency, mean and standard deviation) were used to describe the exact study population. Independent Samples T-test and Binary Logistic Regression was used for analytical statistics and P<0.05 was considered statistically significant.
Results
A total number of 924 subjects were enrolled within 6 months of screening. The mean age of the participants was 59.38±7.15 years. The participation of women (57.0%) and rural residents (54.2%) was higher. Participation rate decreased by increasing the age, and the highest participation rate was observed in the age group less than 60 years. In terms of education level, the highest frequency (51.5%) belonged to elementary to high school group, and 89.4% of subjects were married. In terms of BMI, the most of the cases (44.3%) were in the overweight group. The demographic characteristics of participants are shown in Table 1.
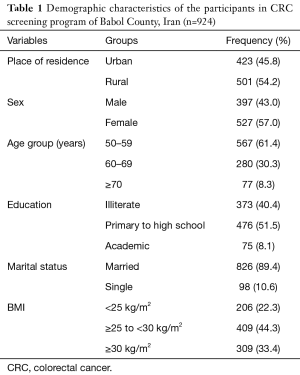
Full table
Among all the studied subjects, 897 cases (97.1%) provided at least two acceptable samples to the laboratory based on the preliminary guidance, in which 229 cases (25.5%) had at least one positive test result that were considered as positive IFOBT in this program. In terms of the differences in demographic characteristics according to the test results, the mean age was different (in the positive test: 60.83±7.20 years versus the negative test: 58.88±7.08 years; P<0.001). Also, the results of regression analysis showed that age was considered as a strong independent predictor among demographic variables. The difference in the test results in age groups was different and significant in the one-way and multivariate regression analysis; so that, the prevalence of the positive test result was 64% higher in the age range of 61–70 years than the age group ≤60-year-old (OR =1.64; 95% CI, 1.15–2.34, P=0.006). Also, the prevalence of the positive test result was more than 2 times higher in subjects over 70 years old than ≤60 years (OR =2.05; 95% CI, 1.18–3.55, P=0.010). In terms of education, in the univariate analysis, the prevalence of positive test result was 50% less in university graduates than illiterates subjects; the difference was statistically significant (OR =0.50; 95% CI, 0.25–0.97, P=0.041). In the presence of other variables, although the prevalence of positive result was 46% less in university graduates than illiterate ones, but the difference was not significant (OR =0.54; 95% CI, 0.26–1.13, P=0.106). The prevalence of IFOBT positive was 13% lower in rural residents than in urban residents, and it was 6% higher in women than in men and 20% higher in obese subjects than normal BMI people. None of these differences were statistically significant (Table 2).
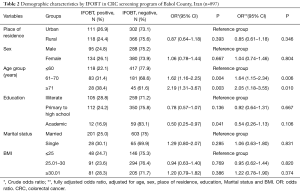
Full table
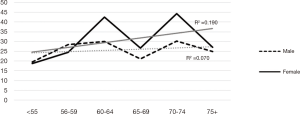
The likelihood of a positive test result (IFOBT) based on age groups of 5 years. The results presented in Table 3 show that the chance of positive test result in the age group ≤55 was 23% less, which was increased by 64% in the age group above 75. The results of the trend test also confirmed this increase by increasing age (P<0.001) (Table 3). In a further investigation in terms of the percentage of positive test results in different age groups based on gender (Figure 1), this trend was significant for both sexes, but the trend test was only statistically significant in women (P<0.001).

Full table
Among 229 patients with positive IFOBT, 118 cases (64.1%) were referred to the hospital for colonoscopy. In examining the differences in the demographic characteristics of the subjects who had positive test results, there was no significant difference between the mean age of the subjects referring to colonoscopy (mean ± SD of colonoscopy cases: 60.50±7.08 years versus non-colonoscopy subjects, 61.18±7.34 years); (P=0.477). Colonoscopy cases compared to non-colonoscopy subjects had a higher proportion in terms of male participants, urban residents, married subjects, and obese individuals. Although there are minor differences, none of them were statistically significant (P>0.05). The results presented in Table 4 show that the demographic characteristics in the two groups were similar and the results of screening in the colonoscopy group could be generalized to the non-colonoscopy group. In this study, three cases of CRC and 28 polyps were identified from colonoscopy group.
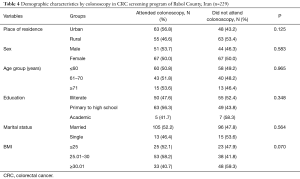
Full table
Discussion
This study is the first estimate of CRC screening on the demographic characteristics in the general population for people at moderate risk who aged over 50 years in northern part of Iran (Babol). The results of this study showed that the participation of women and rural residents in the screening program was higher and the participation rate was declined by increasing the age. Among the demographic variables in the results of the screening test, age was considered as a strong independent predictor for positive IFOBT. Also, there was no difference in terms of demographic characteristics between those who attended colonoscopy and those who not.
Participation is a key indicator of the success for any program. After completing the questionnaires, 97.1% of subjects had at least two acceptable samples to the lab based on the initial guidelines. In patients with positive test results, about two-thirds of them were referred to the hospital for colonoscopy. In Iran, a cross-sectional study by Mirzaei et al. reported that on the evaluation of CRC screening program (screening for relatives of patients), the rate of primary participation and colonoscopy cases were 53.3% and 54.8%, respectively. Their target group was the first-degree relatives who were at the higher risk (17). In a population-based cross-sectional study entitled CRC screening behaviors and related factors in the elderly population of China (over 50 years old) who were invited by phone calls, the participation rate was 66.6%, and the rate of attending the IFOBT and colonoscopy tests were 19% and 12%, respectively (18). In a CRC screening program in UK in the first stage, 56.7% of IFOBT and 82.7% of those who had positive test results were participated in the colonoscopy program (19). In the study by Mansouri et al in Iran, 76% of participants who had positive test result performed the colonoscopy (20). In a Canadian study, which was the first screening program in five provinces, colonoscopy participation was 80.2% (21). The difference in program coverage for different countries could be due to differences in the program’s target group, and the performing and sampling methods. Considering the invasive nature of the colonoscopy, the participation rate in the IFOBT screening program is significantly higher than colonoscopy (22), which is consistent with the results of present study.
Based on the results of present study, the participation of women and rural residents in the screening program was higher and the participation rate was declined by age. Previous studies indicated that male gender, older age, marital status, higher education, higher income, urban residence, and ethnicity were identified as screening attendance predictors among the demographic characteristics (15,16). In a cross-sectional study on the results of the first CRC screening program in Mexico, 74% of the participants were female (19), which is consistent with the results of present study. In the Mansouri study in Iran, fewer screening was associated with younger age, male gender, and social exclusion (20).
Participation rate in the first stage of screening was high in our study, which could be due to sampling method, and in the second stage –the colonoscopy- the participation rate was higher than the other study in Iran and to some extent was equal to studies of other countries, which can be due to ongoing follow-up during the program.
Except the study by Mirzaei in Iran, colonoscopy was performed in the second phase and for people who had positive IFOBT in all the studies of other countries. In some studies of other countries, kits were sent to individuals by mail. It should be noted that the most important barrier to reduce CRC mortality is the low participation in screening (23). The benefits of screening will only be high in the community once the participation is high. Moreover follow-up is an important matter during the program and colonoscopy (18). Barriers for CRC screening can be the lack of information, bad feeling about doing the test, and lack of proper justification by the doctor. So, increasing the community’s awareness and changing attitudes of the people, especially about colonoscopy, can increase the level of participation. All these demographic characteristics should be considered in planning a screening program.
Among demographic variables, age was considered as a strong independent predictor in IFOBT positive result. A study in the United States in which 10,702 individuals were participated in the study within a year, showed that 75.7% had at least one sample and 16.2% had at least one positive result and the rate of positivity was increased by age (24). In the Mansouri study, both men and older men were more likely to have positive tests; so that, the prevalence of positive test was 2.07 times higher in people ≥65 years old compared to those less than 55 years old (20). In general, positivity was increased by age, which is consistent with the results of this study. In our study, the prevalence of positive result in women was 6% higher, but in the study of Mansouri, 43% were lower in women (20), which is not consistent with the results of our study. Positive test result does not depend solely on the demographic characteristics and can be due to the different target groups, the program implementation, and many other factors that need to be investigated in other studies.
In the present study, for the subjects with positive test results, colonoscopy was performed. There was no statistically significant difference between colonoscopy and non- colonoscopy subjects in terms of demographic characteristics: males, urban residents and marital status. Men were tended to perform colonoscopy more than women. Perhaps it’s because women think that CRC is less important than other sexual cancers, such as breast cancer. Women may also have misunderstood that CRC is a male disease (25).
In the present study, performing colonoscopy more in urban residents was also due to the quicker and easier access to hospital centers; it should be considered in screening program that rural residents should also have easy access to colonoscopy centers. Observing more married cases can also be justified by more support in the family.
The strength point of this study was that this was the first screening program in terms of investigating the demographic characteristics in asymptomatic individuals at moderate risk. This study also had limitations, including the small sample size and the fact that our sample cannot be representative of the population as a whole because of available sampling, participation of people over 50 years old, asymptomatic individuals and considering inclusion and exclusion criteria, or may be due to not considering all the potentially influencing factors on CRC. These features should be considered in interpreting the findings. Also, our analysis was based on one screening program. Longitudinal studies are needed to determine the complete screening behavior of people in terms of demographic characteristics. Due to the lack of the similar studies in the country, it is recommended to conduct similar and complementary researches in other areas with more populations to achieve better conclusion from the total results. Besides, health education and health promotion programs should also focus more on these identified factors to facilitate the screening of CRC in the population; based on the results of this study the screening of asymptomatic people over 50 in the general population should also be prioritized.
Acknowledgements
The assistance and cooperation of Health Deputy of Babol University of Medical Sciences and all the staff of health centers who helped us in data collection process are highly appreciated.
Funding: A substantial part of this study was supported by Research Council of Shiraz University of Medical Sciences for which we are thankful (Grant Number: 95-01-42-13948).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by ethics committee of Shiraz University of Medical Sciences (No. IR.SUMS.REC.1396.S5807). Informed consent was obtained from all patients.
References
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [Crossref] [PubMed]
- National Cancer Registry Report 2003–2004. Tehran, Iran: Ministry of Health, Deputy for Health Directory, CDC Cancer Office 2004 (Persian). Available online: http://ircancer.ir
- Center MM, Jemal A, Smith RA, et al. World wide variations in colorectal cancer. CA Cancer J Clin 2009;59:366-78. [Crossref]
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; Available online: http://globocan.iarc.fr
- Nikbakht HA, Aminisani N, Asghari Jafarabadi M, et al. Trends in the incidence of colorectal cancer and epidemiologic and clinical characteristics of survivors in Babol City in 2007-2012. J Babol Univ Med Sci 2015;16:7-14.
- Minozzi S, Armaroli P, Segnan N. European guidelines for quality assurance in colorectal cancer screening and diagnosis. First Edition--Principles of evidence assessment and methods for reaching recommendations. Endoscopy 2012;44 Suppl 3:Se9-14.
- Winawer SJ. The multidisciplinary management of gastrointestinal cancer. Colorectal cancer screening. Best Pract Res Clin Gastroenterol 2007;21:1031-48. [Crossref]
- Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000;343:1603-7. [Crossref] [PubMed]
- Towler B, Irwig L, Glasziou P, et al. A systematic review of the effects of screening for colorectal cancer using the faecal occult blood test, hemoccult. BMJ 1998;317:559-65. [Crossref]
- Lee CS, Ronan L, O'Morain C, et al. Screening for colorectal cancer: what fits best? Expert Rev Gastroenterol Hepatol 2012;6:301-12. [Crossref] [PubMed]
- Stockbrugger R. Isolated colorectal cancer screening or integrated cancer prevention? A provocative suggestion! Dig Dis 2012;30:316-9. [Crossref] [PubMed]
- Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale-Update based on new evidence. Gastroenterology 2003;124:544-60. [Crossref] [PubMed]
- Shelton RC, Jandorf L, Ellison J, et al. The influence of sociocultural factors on colonoscopy and FOBT screening adherence among low-income Hispanics. J Health Care Poor Underserved 2011;22:925-44. [Crossref] [PubMed]
- Fenton JJ, Tancredi DJ, Green P, et al. Persistent racial and ethnic disparities in up-to-date colorectal cancer testing in medicare enrollees. J Am Geriatr Soc 2009;57:412-8. [Crossref] [PubMed]
- Mobley L, Kuo TM, Urato M, et al. Predictors of endoscopic colorectal cancer screening over time in 11 states. Cancer Causes Control 2010;21:445-61. [Crossref] [PubMed]
- Guessous I, Dash C, Lapin P, et al. Colorectal cancer screening barriers and facilitators in older persons. Prev Med 2010;50:3-10. [Crossref] [PubMed]
- Mirzaei H, Panahi M, Etemad K, et al. Evaluation of Pilot Colorectal Cancer Screening Programs in Iran. Iranian Journal of Epidemiology 2016;12:21-8.
- So WK, Choi KC, Chan DN, et al. Colorectal cancer screening behaviour and associated factors among Chinese aged 50 and above in Hong Kong - a population-based survey. Eur J Oncol Nurs 2012;16:413-8. [Crossref] [PubMed]
- García-Osogobio S, Téllez-Ávila FI, Méndez N, et al. Results of the first program of colorectal cancer screening in Mexico. Endoscopia 2015;27:59-63. [Crossref]
- Mansouri D, McMillan DC, Grant Y, et al. The impact of age, sex and socioeconomic deprivation on outcomes in a colorectal cancer screening programme. PLoS One 2013;8:e66063. [Crossref] [PubMed]
- Major D, Bryant H, Delaney M, et al. Colorectal cancer screening in Canada: results from the first round of screening for five provincial programs. Curr Oncol 2013;20:252-7. [Crossref] [PubMed]
- Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012;366:697-706. [Crossref] [PubMed]
- Sano Y, Byeon JS, Li XB, et al. Colorectal cancer screening of the general population in East Asia. Dig Endosc 2016;28:243-9. [Crossref] [PubMed]
- Allison JE, Tekawa IS, Ransom LJ, et al. A comparison of fecal occult-blood tests for colorectal-cancer screening. N Engl J Med 1996;334:155-9. [Crossref] [PubMed]
- Meissner HI, Breen N, Klabunde CN, et al. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev 2006;15:389-94. [Crossref] [PubMed]


