Comparison of adiponectin concentration between pancreatic cancer and colorectal cancer
1University of Saint Etienne, Saint Etienne, France; 2Department of Endocrinology, University Hospital, Grenoble, France
|
Original Article
Comparison of adiponectin concentration between pancreatic cancer and colorectal cancer
1University of Saint Etienne, Saint Etienne, France; 2Department of Endocrinology, University Hospital, Grenoble, France
|
|
Abstract
Introduction: Adiponectin (ADP) is an adipocytokine secreted by the adipose tissue which can be a useful marker in oncogenesis. Preliminary studies suggest that adiponectin rates differ according to the type of cancer.
Aim of study: Compare ADP plasma levels in pancreatic cancer (PC) and colorectal cancer (CRC) in a prospective monocentric study.
Patients and methods: The study included all the incident cases of PC gathered from a university hospital in France
from January 2006 till September 2007. A control population of incident cases of colorectal cancer (CRC), matching or
age, gender, and tumor staging was set in the same period. In addition to demographic data, the other parameters analyzed were: ADP rate, insulinoresistance (Homa-test), presence of a dysmetabolic syndrome, evolution of weight and
data concerning the tumor (staging, tumor markers: ACE, CA19.9).
Results: 33 CRC and 53 PC were analyzed. Type 2 diabetes was found in 18.2% of the CRC cases and 39.6% of the PC
(p = 0.037). The mean ADP level was significantly higher in PC versus CRC (20.9 microgram/l versus 15.9 microgram/l;
p = 0.03). In multivariate analysis , after adjusting for gender, age, bilirubinemia and weigth loss, the variables independently associated with a high level of ADP (> 10 microG/L) were type 2 diabetes (OR = 0.05, p = 0.01), insulinoresistance (OR = 0.42, p = 0.05) and PC (OR = 12.03, p = 0.047).
Conclusion: ADP concentration is higher in PC patients than in CRC patients. ADP concentration > 10 microgram/l was independently associated with pancreatic cancer. Our data confirm that adiponectin rates differ strongly according to the type of cancer.
Key words
pancreas cancer, colorectal cancer, mellitus diabetes, adiponectin
J Gastrointest Oncol 2011; 2: 232-239. DOI: 10.3978/j.issn.2078-6891.2011.031
|
|
Introduction
In Western countries pancreatic cancer represents the
fourth cause of cancer death and its incidence rates, between
6 and 10 per 100000 populations, has increased in the last
30 years.
In 2007, in the United States, pancreatic cancer was responsible for one out of 75 deaths caused by cancer (1).
Prognostic is very modest with an overall 5 years survival
rate at less than 4%, the lowest of all solid tumours. Medical
or surgical palliative treatment can significantly increase the
comfort of life, but only modestly increases survival.
Only in a subset of patients, with T1 tumour (TNM
classification), resectional surgery can be curative, with a 5
year survival rate reported was 20% (3).
Adiponectin is an adipokin product of mature adipocyte,
reduced in the case of insulin resistance and positively
correlated with insulin sensitivity. Adiponectin regulates
intracellular pathways of protein kinase activated by AMP
(AMP-kinase), of c-JUN and c-JUN N-terminal kinase
(JNK) and of the signal that transcribes and activates
transcription 3 (STAT3). Therefore, adiponectin is an antiinflammatory,
anti-angiogenic and a block for cell growth.Circulating concentrations of adiponectin are inversely
correlated to the risk of several cancers: breast cancer (4),
endometrium (5), prostate (6), clear cell cancer kidney (7),
stomach cancer (8) and leukemia (9). Prospective studies
have shown that there is, at distance, a major risk of breast
cancer (10), endometrial (11) and colo-rectal cancer (12) in
postmenopausal women if adiponectin serum level is low.
Adiponectin present a direct antitumor (13) and
proapoptotic effect. Conversely, in pancreatic cancer, results
about ADP are conflicted (14,15).
The principal aim of our study was to compare ADP
concentrations in two groups of cancer (colorectal cancer
and pancreas cancer) matched on age, sex and tumour
staging (metastatic or non metastatic).
|
|
Patients and methods
This prospective study included all consecutive patients
with a new diagnosis of pancreatic adenocarcinoma followed
in a referent university hospital between January 2006 and
September 2007. The control group included patients with
new diagnosis of colorectal carcinoma diagnosed in the
same period and matched for sex, age and tumour staging
(metastatic or non metastatic tumour), according to the
sixth edition of American Joint Committee on Cancer:
tumour, node, metastasis (TNM) classification system.
In all cases diagnosis was histological or cytological.
All patients were informed and signed a consent paper.
Patients on chemotherapy or on antidiabetic treatment were
excluded from the study. All patients were characterized by
age, sex, body mass index (BMI) before and at the moment
of diagnosis, the presence of diabetes according to the
criteria of the American Diabetes Association.
When diabetes was pre-existing, we evaluated the
interval between diagnosis of diabetes and diagnosis of
pancreatic cancer.
We noted a family history of diabetes, and the presence
or absence of an associated dysmetabolic syndrome:
hypertension, dyslipidemia, obesity.
Tumour data were: stage, size and tumour markers
(CEA and CA 19-9); patients were divided into two groups:
resectable cancer or locally advanced/metastatic.
Clinical Chemistry
Folate and vitamin B12 were assessed at the time of
inclusion into the study. The HOMA index was calculated
after the dosage of insulin.
Adiponectin level
All biological samples were harvested in the morning
before breakfast, and the serum was immediately separatedby centrifugation and stored at -80°C until dosage was
completed.
This process was completed with recombinant human
adiponectin by standard (Human Adiponectin RIA Linco
Research® 6 research Park Dr St Charles, Missouri 63304
USA) using the instructions of manufacturer.
Statistical analysis
Statistical analysis was performed by using SPSS software
(version 11, SPSS Inc, Chicago, IL, USA). Quantitative
variables were expressed as median and range, or as mean ±
standard deviation when normally distributed. Parametric
student’s test or non parametric Mann-Whitney’s test when
appropriate were used to compare quantitative variables
between the 2 groups. The relationship between the type
of cancer and the other variables, especially the presence of
diabetes and the rate of adiponectin was analyzed using χ2
test. A p value less than 0.05 was considered to indicate a
significant difference.
The threshold of adiponectin level was investigated by
analysis of ROC curves and measuring the areas under the
curves for a better sensitivity and specificity.
For multivariate analysis, we used binar y logistic
regression to find the independent factors significantly
associated with adiponectin level (low or high comated
with a threshold level of ADP) and diabetes with pancreatic
cancer.
The variables were analyzed in the multivariate model
for a risk α < 10%. Values of p < 0.05 were considered
statistically significant.
|
|
Results
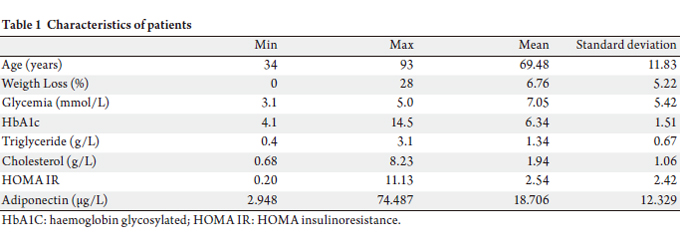
Characteristics of patients
Between January 2006 and September 2007, 53 consecutive
patients with pancreatic adenocarcinoma and 30 with
colorectal adenocarcinoma were analyzed. Mean age for
the two groups was 69 years (range, 11.9 years). The mean
HOMA index was 2.54 and the mean adiponectin level was
18.7 μg/L (range 2.9-74.5).
The main demographic and clinical characteristics of all
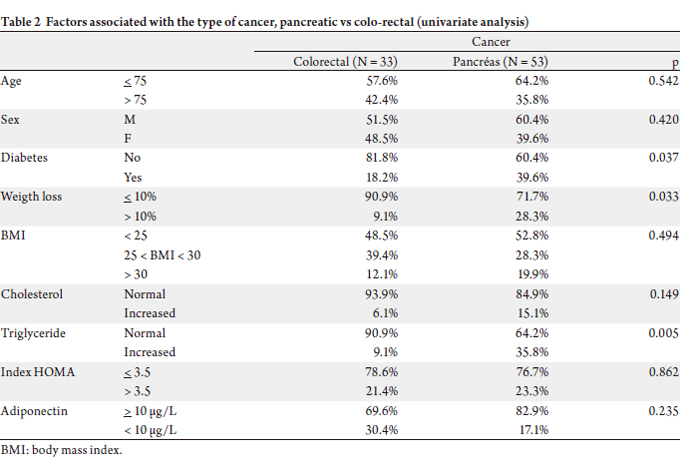
included patients are presented in Table 1. Table 2 shows the
factors associated with the type of cancer. The two groups
(pancreatic cancer and colorectal cancer) were comparable
for age, sex, BMI, the rate of cholestérol and tumour staging.
In the pancreatic group there was however an increased
incidence of hypertriglyceridemia (35.8% vs 9.1%, p = 0.05).
Pancreatic cancer was associated with severe weight loss
(BMI < 20) in 1/3 of the cases against 1/10 in the second
group. At the moment of diagnosis, diabetes was two times
more frequent in the group of patients with pancreatic cancer compared to patients presenting with colorectal
cancer (39.6% vs 18.2%, p = 0.037).
  Adiponectin rate
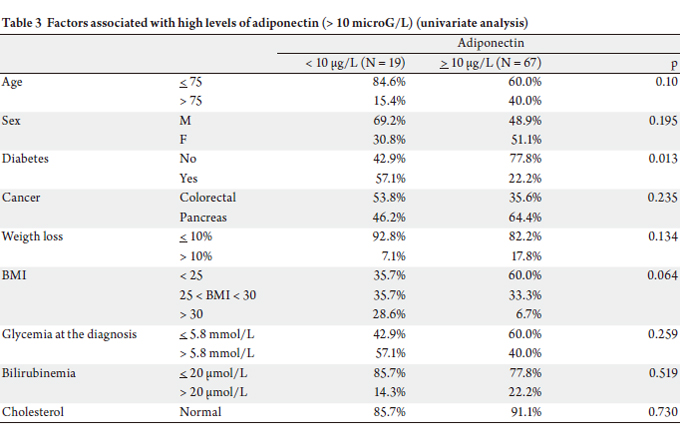
The mean level of adiponectin was significantly different
by univariate analysis (Table 3): between the two groups
(20.95 g/L in pancreatic cancer group versus 15.98 g/L in
the colorectal cancer, p = 0.03).
After analyzing the ROC curves in the PC group, we
selected as threshold a rate of adiponectin of 10 μg/L, with the best sensitivity/specificity ratio for the association
between high ADP level and PC. The area under the receiver
operating characteristic curve (ROC) for the highest ADP
concentration was 0.81 (OR = 21.1; 95%CI = 1.4-150; p =
0.031). A specificity of 87% was seen at the cut-off level of
10 microG/L but with a sensitivity of 75%. In this study, the
threshold value could be part of the diagnosis of pancreatic
cancer in diabetes mellitus, with a sensibility of 87%.
There was no significant difference between both groups
in univariate analysis for the portion of patients above this threshold (adiponectin > 10 μg/L: 69.6% vs 82.9%, p =
0.195). The HOMA indexes were comparable between the
two groups.
In the pancreatic cancer group, adiponectin levels were
lower (less than 10 g/L) in the presence of type 2 diabetes
(44.4% vs 14.6%, p = 0.013) and in the presence of insulin
resistance measured by HOMA index (50.0% vs 11.5%, p =
0.049).
In multivariate analysis (Table 4) , after adjustment on
sex, age ( 20 μmol/L) and weight
loss (> 10%), the variables independently associated with
high levels of adiponectin (> 10 μg/L) were : the presence
of pancreatic cancer (OR = 12.03, p = 0.047), diabetes (OR
= 0.07, p = 0.01) and the insulin resistance (OR = 0.42, p =
0.05).
In conclusion, adiponectin is twelve times higher (> 10
μg/L) in patients presenting with pancreatic cancer than in
patients with colorectal cancer after adjustment on diabetes
mellitus (Table 4).
  Adiponectin-Diabetes Relationship
The low number of diabetic patients in the colorectal
cancer group has not allowed analysis and comparison
with the group with pancreatic cancer. We therefore
focused on the characterization of diabetes in patients with adenocarcinoma of the pancreas. Diabetes was present in
21 patients (39.6%) with pancreatic cancer. It was present
of PC within 3 months before diagnosis in 34% of cases and
in 43.0% of cases within 3 years preceding the diagnosis
of pancreatic cancer. One half of patients were men (p =
0.857).
The age at the time of diagnosis of pancreatic cancer
was not statistically different according to the presence
or absence of diabetes. Diabetic patients under 75 years
represent 59.3% of cases (p = 0.760).
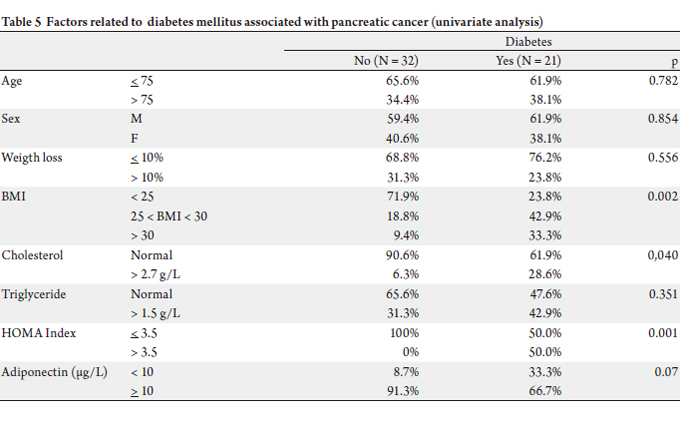
In univariate analysis, the presence of diabetes was
associated with obesity (over-weight : 42.9% vs 18.8%,
obesity : 33.3% vs 9.4%, p = 0.002), hypercholesterolemia
(28.6% vs 6.3%, p = 0.037) and insulin-resistance (HOMA
> 3.5 : 50.0% vs 0%, p = 0.001).
Non-diabetic patients did not show insulin resistance;
55% of diabet ic pat ients presented a HOMA index
higher than 3.5. An adiponectin rate < 10 μg/L was not
statistically linked to type 2 diabetes, but there was
a trend because 33.3% of diabetic patients had lower
adiponectin levels (only 8.7% of non diabetics, with p =
0.07) (Table 5).
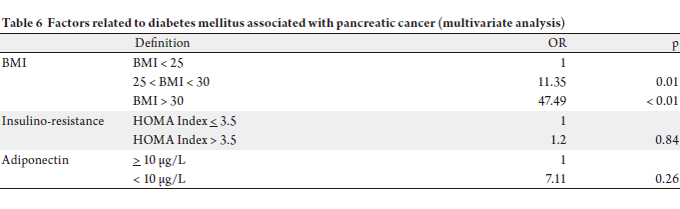
In multivariate analysis, only obesity was an independent
factor explaining diabetes (Overweight: OR = 11.35, p =
0.01, obesity: OR = 47.49, p < 0.01). The insulin-resistance and adiponectin level’s < 10 μg/L were not statistically
associated with diabetes (respectively OR = 1.2, p = 0.84
and OR = 7.11, p = 0.26) (Table 6).
  |
|
Discussion
Our study confirms that adiponectin level is variable
with the type of cancer; and demonstrates that the mean
level of ADP is significantly higher in PC than in CRC.
In multivariate analysis, ADP concentration of up to 10
microG/l was independently associated with PC. For the
first time our results show that serum adiponectin level is 12
times higher in pancreatic cancer than in colorectal cancer.
Published studies showed an inverse correlation
between plasma levels of adiponectin and incidence of
different cancers (4-9) probably because adiponectin could
have an antitumor action through a pro-apoptotic and
antiangiogenic pathway.
Data about the association between ADP and colorectal
tumours are in agreement with that. In a recent cross
sectional study, Okate et al (16) concluded that a decreased
level of adiponectin was strongly associated with an
increased risk of colorectal adenoma and early cancer but
not with advanced cancer. The threeshold level of ADP in
this study is comparable to our results (11 microG/l). If
we don’t demonstrate that the mean of ADP in the CRC
is low (15.9 microG/l); more than one third of this group
of patients presented an ADP under 11 microG/l. All the
patients included presented an advanced cancer in our
population. In a case control study, Gonullu et al (17)
reported that adiponectin level was negatively correlated
with a CRC and with the stage of the cancer. In this study,
adiponectin could be responsible for a poor prognosis in
colorectal cancer. Moreover serum adiponectin level seem
negatively associated with higher risk of colorectal cancer
and cancer stage and grade (18,19). In these two recent
studies expression of adiponectin receptors was significantly
stronger in adenocarcinoma than in normal tissue.
The association between adiponectin and pancreatic
cancer is, conversely, more debated. For the first time,
Chang et al. (14) reported a significant increase of ADP
concentration in patients with operable pancreatic cancer
compared to patients with chronic pancreatitis and the
control group. In this study, the ADP test used is different
from our study, so the threshold isolated cannot be
extrapolated. In a case control study, Dalamaga et al (20)
demonstrated that higher adiponectin levels were associated
with PC (p 21) conducted a
nested case control study. They demonstrate that higher
ADP concentration (> 10 microG/l) were inversely
associated with pancreatic cancer (OR = 0.65; p = 0.04). The
inverse association was significant among cases diagnosed 5
or more years after blood concentrations.
Another recent nested-case control study observed a
non-significant decrease in pancreatic cancer risk with
higher adiponectin serum levels (22.) In our study, the ADP
level was determined in patients with a proved PC, many
of them presenting with weight loss and high inflammatory
status. So, we can explain in part the paradigm.
Our study does not allow demonstrating directly if the
ADP level is low in CRC or high in PC because we did not
compare our cancer groups with a healthy control group.
However, we consider that the level in patients with PC is
high. One of our explanations is linked to diabetes mellitus.
Pancreatic adenocarcinoma is the cancer most often
associated with type 2 diabetes and/or metabolic syndrome,
up to 80% in some series (15). It is well recognized that
the reduction of adiponectin level in serum is involved
in the genesis and the aggravation obesity and type 2
(16). The existence of tumor disease and association of
diabetes (in pancreatic cancer) are two factors that should
be associated with a significant decrease of adiponectin
rate. Our results are concordant with those of Chang et al.
(14) : they discovered high levels of adiponectin in patients
with pancreatic cancer compared to subjects with chronic
pancreatitis or healthy.
For the first time our results demonst rate that
adiponectin level is 12 times higher in pancreatic cancer
than in colorectal cancer (control group in our study).
So, we can speculate that adiponectin doesn’t present an
antineoplasic property in PC or that diabetes mellitus could
be the explanation for the difference of adiponectin in
PC. The time between the onset of diabetes and diagnosis
of pancreatic cancer is 3 years or less in 43% of cases in
our study. This short period does not suggest a causal link
between a classic diabetes mellitus and neoplastic disease,
as has been described for type 2 “classic” diabetes in general
(17, 20). There is certainly a different mechanism linking
type 2 diabetes and pancreatic cancer. The most probable
mechanism is that diabetes is a direct consequence of
pancreatic cancer through a biochemical or mechanical
lower insulin levels. Some works have suggested that
diabetes associated with pancreatic cancer would be
consequence of alterations of insulin secretion in beta
cells (21,23). In our study, among diabetic patients with
pancreatic cancer, there was a context of insulin resistance
in only 50% of the cases; incidence of diabetes was two times higher than in control group (38% vs 19%). The frequency
of diabetes in the control group was comparable to type 2
diabetes in general population (prevalence between 10 and
20% after 35 years). All diabetics in colo-rectal cancer group
had an insulin-resistance, characteristic of “classic” type 2
diabetes. This observation suggests that in pancreatic cancer
group, 50% of mellitus diabetes was “classic”, and 50% of
others types of diabetes, directly associated with pancreatic
cancer and probably linked to insulin deficiency.
Some weaknesses can be reported in our study. We have
not included a healthy group. We have chosen to compare
two different tumour populations rather than using a
control group of healthy subjects because we wanted to
validate the divergent evolution of adiponectin rate during
theses cancers. Because of the relatively small population
in our study we could not possibly explore in detail the
subgroup of diabetic patients and our odds ratio, have wide
confidence intervals and are only informative. Our article
is a transversal study that allows evaluation of adiponectin
rate at the moment when cancer becomes symptomatic,
so we can not evaluate the kinetics of adiponectin before
apparition of neoplasia. At last, there isn’t a consensus
between manufacturers of kits for the determination of
adiponectin (positivity or increased serum level). The
case-controlled studies conducted in various cancers have
showed variable rates. The rate of adiponectin was often less
than 9 μg/L in cancer cases, and generally between 10 μg/L
and 14 μg/L in the control group without cancer. In the
study by Chang et al. comparing the rates of adiponectin
in pancreatic cancer (14), in chronic pancreatitis and in
healthy subjects, the averages were respectively 21.1, 13.7
and 5.8 μg/L. After the analysis of ROC curves, we have
chosen 10 μg/L as the threshold of positivity, but this must
be confirmed by further studies with a larger numbers of
patients.
|
|
Conclusion
In summary, we demonstrate that adiponectin
concentration is higher in PC than in CRC. Our results
confirm indirectly that in CRC, adiponectin is often low
and higher in pancreatic cancer. We demonstrated that
diabetes could be a factor for PC and differ in function of
the natural in PC. Our data can speculate that we have
two different mechanisms of natural history on PC. So,
we hypothesize that an old diabetes mellitus could be an
moderate risk factor of PC associated within an increase
of IGF level and low adiponectin concentration and
conversely an early diabetes with insulopenia and high level
of adiponectin secondary and witness of an new PC. So, we
think that other prospective studies must control our results and analyse the real key of adiponectin in these tumors.
|
|
References
Cite this article as:
Phelip J, Bageacu S, Baconnier M, Barabino G, Tedesco E, Benhamou P, Roblin X. Comparison of adiponectin concentration between pancreatic cancer and colorectal cancer. J Gastrointest Oncol. 2011;2(4):232-239. DOI:10.3978/j.issn.2078-6891.2011.031
|