|
Cite this article as:
Khokhlova T, Hwang J. HIFU for palliative treatment of pancreatic cancer. J Gastrointest Oncol. 2011;2(3):175-184. DOI:10.3978/j.issn.2078-6891.2011.033
Review Article
HIFU for palliative treatment of pancreatic cancer
Tatiana D. Khokhlova, Joo Ha Hwang
Division of Gastroenterology, Department of Medicine, Center for Industrial and Medical Ultrasound, Applied Physics Laboratory, University of
Washington, Seattle, WA, USA
Corresponding author: Joo Ha Hwang, MD, PhD. University of Washington,
1959 NE Pacific Street, Box 356424, Seattle, WA 98195. Tel: 206-685-2283; Fax:
206-221-3992. E-mail: jooha@medicine.washington.edu
|
|
Abstract
High intensity focused ultrasound (HIFU) is a novel non-invasive modality for ablation of various solid tumors including
uterine fibroids, prostate cancer, hepatic, renal, breast and pancreatic tumors. HIFU therapy utilizes mechanical energy
in the form of a powerful ultrasound wave that is focused inside the body to induce thermal and/or mechanical effects in
tissue. Multiple preclinical and non-randomized clinical trials have been performed to evaluate the safety and efficacy of
HIFU for palliative treatment of pancreatic tumors. Substantial tumor-related pain reduction was achieved in most cases
after HIFU treatment, and no significant side-effects were observed. This review provides a description of different physical
mechanisms underlying HIFU therapy, summarizes the clinical experience obtained to date in HIFU treatment of
pancreatic tumors, and discusses the challenges, limitations and new approaches in this modality.
Key words
Therapeutic ultrasound; focused ultrasound; HIFU; pancreas cancer; review
J Gastrointest Oncol 2011; 2: 175-184. DOI: 10.3978/j.issn.2078-6891.2011.033
|
|
Introduction
Within the last year more than 42,000 people in the United
States were newly diagnosed with pancreatic cancer, which
makes it the fourth leading cause of cancer mortality ( 1). A
majority of patients diagnosed with pancreatic cancer are
considered inoperable at the time of the diagnosis due to
locally advanced disease or the presence of metastasis, and
the efficacy of systemic chemotherapy is limited ( 2). The
prognosis for these patients is one of the worst among all
cancers: according to EUROCARE study, based on over
30,000 cases, overall survival at 1,3 and 5 years was 16%, 5%
and 4%, respectively ( 3). Pain is often reported by patients
with advanced disease, and palliative treatment methods are
commonly employed and include opioid therapy and celiac
plexus neurolysis ( 4). However, opioids may produce a range
of side-effects from dysphoria to respiratory depression, and celiac plexus neurolysis provides limited benefit in pain
relief, in addition to being an invasive procedure ( 5, 6). High intensity focused ultrasound (HIFU) therapy is a
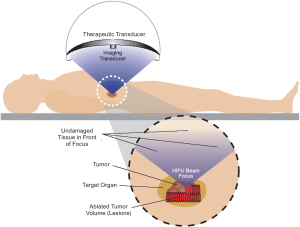
non-invasive ablation method, in which ultrasound energy
from an extracorporeal source is focused within the body to
induce thermal denaturation of tissue at the focus without
affecting surrounding organs ( Figure 1). HIFU ablation
has been applied to treatment of a wide variety of both
benign and malignant tumors including uterine fibroids,
prostate cancer, liver tumors and other solid tumors that are
accessible to ultrasound energy ( 7-10). Preliminary studies
have shown that HIFU may also be a useful modality for
palliation of cancer-related pain in patients with advanced
pancreatic cancer ( 11-14). The objective of this article
is to provide an overview of the physical principles of
HIFU therapy and to review the current status of clinical
application of HIFU for pancreatic cancers.
|
|
Physical mechanisms underlying HIFU therapy
Ultrasound is a form of mechanical energy in which waves
propagate through a liquid or solid medium (e.g., tissue)
with alternate areas of compression and rarefaction. The
main parameters that are used to describe an ultrasound
wave are its frequency, or the number of pressure oscillations
per second, and pressure amplitude, as illustrated in Figure
2C. Another important characteristic of an ultrasound wave is its intensity, or the amount of ultrasound energy per unit
surface, which is proportional to the square of the wave
amplitude. Both HIFU devices and diagnostic ultrasound imagers
utilize ultrasound waves with frequencies t ypically
ranging from 0.2–10 megahertz (MHz), but the difference
is in the amplitude and in how the ultrasound waves are
transmitted. Diagnostic ultrasound probes transmit plane
or divergent waves that get reflected or scattered by tissue
inhomogeneities and are then detected by the same probe.
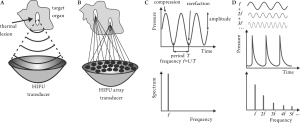
In HIFU the radiating surface is usually spherically curved,
so that the ultrasound wave is focused at the center of
curvature in a similar fashion to the way a magnifying
lens can focus a broad light beam into a small focal spot
( Figure 2A). This can result in amplification of the pressure
amplitude by a factor of 100 at the focus. Another method
of focusing is using ultrasound arrays, as illustrated in
Figure 2B: each element of the array radiates a wave with
a pre-determined phase, so that waves from all elements
interfere constructively only at a desired focal point. The
size and shape of the focal region of most clinically available
transducers is similar to a grain of rice: 2-3 mm in diameter
and 8-10 mm in length. As mentioned above, diagnostic ultrasound and HIFU
waves differ in amplitude. Typical diagnostic ultrasound
transducers operate at the pressures of 0.001 – 0.003
MPa which corresponds to time-averaged intensity of 0.1-100 mW/cm2. HIFU transducers produce much larger
pressure amplitudes at the focus of the transducer: up to 60
MPa peak compressional pressures and up to 15 MPa peak
rarefactional pressures, which corresponds to intensities
of up to 20000 W/cm2. For comparison, one atmosphere is
equal to 0.1 MPa. Ultrasound of such intensities is capable
of producing both thermal and mechanical effects on tissue,
which will be discussed below.
Tissue heating
The fundamental physical mechanism of HIFU, ultrasound
absorption and conversion into heat, was first described
in 1972 ( 15). Absorption of ultrasound, the mechanical
form of energy, in tissue is not as intuitive as absorption
of electromagnetic radiation (e.g., light or RF radiation)
and can be simplistically explained as follows. Tissue can
be represented as viscous f luid contained by membranes.
When a pressure wave propagates through the tissue, it
produces relative displacement of tissue layers and causes
directional motion or microstreaming of the fluid. Viscous
friction of different layers of fluid then leads to heating ( 16). Both diagnostic ultrasound and HIFU heat tissue,
however, since the heating rate is proportional to the
ultrasound intensity, the thermal effect produced by
diagnostic ultrasound is negligible. In HIFU the majority of
heat deposition occurs at the focal area, where the intensity
is the highest. The focal temperature can be rapidly increased causing cell death at the focal region. A threshold
for thermal necrosis, the denaturing of tissue protein, is
calculated according to the thermal dose (TD) formulation:
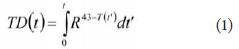 where t is treatment time, and R = 0.25 if T(t) < 43
°C and 0.5 otherwise ( 17). The thermal dose required
to create a thermal lesion is equivalent to the thermal
dose of a 240-min exposure at 43 οC, hence the common
representation of thermal dose in “equivalent minutes”. This
definition originated from the hyperthermia protocol, when
the tissue was heated to a temperature of 43–45°C during a
long exposure of several hours. However, it has been shown
that this model gives good estimations of the thermal
lesion dose for the higher temperatures caused by HIFU.
For example, thermal lesion forms in 10 s at 53°C and 0.1 s
at 60°C. In HIFU treatments, the temperature commonly
exceeds 70°C in about 1–4 s. Thus, tissue necrosis occurs
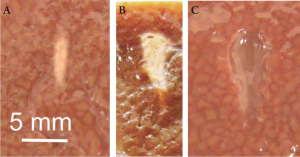
almost immediately. Figure 3A shows an example of a lesion
with coagulation necrosis after a single treatment with a 1
MHz HIFU device in ex vivo bovine liver. It is worth mentioning here that ultrasound absorption in
tissue increases nearly linearly with ultrasound frequency;
hence, more heating occurs at higher frequencies. However, the focus becomes smaller with higher frequency ( 18), and
penetration depth is also limited by the higher absorption.
Therefore, HIFU frequency should be chosen appropriately
for smaller and shallower targets or larger targets located
deeper within the body. In most applications that utilize the thermal effect of
HIFU the goal is to induce cell necrosis in tissue from
thermal injury. However, several studies have reported that
HIFU can also induce cell apoptosis through hyperthermia,
i.e. sub-lethal thermal injury ( 19). In apoptotic cells, the
nucleus of the cell self-destructs, with rapid degradation of
DNA by endonucleases. This effect may be desirable in some
cases, but may also present a limitation for HIFU ablation
accuracy. Since cell death due to apoptosis occurs at lower
thermal dose than thermal necrosis, the tissue adjacent to
the HIFU target might be at risk from this effect ( 20).
Acoustic cavitation
Acoustic cavitation can be defined as any observable
activity involving a gas bubble(s) stimulated into motion
by an exposure to an acoustic field. The motion occurs in
response to the alternating compression and rarefaction
of the surrounding liquid as the acoustic wave propagates
through it. Although live tissue does not initially contain
gas bubbles, tiny gas bodies dispersed in cells may serve as cavitation nuclei that grow into bubbles when subjected
to sufficiently large rarefactional pressure that “tears” the
tissue apart at the site of a nucleus. Thus, cavitation activity
in tissue may occur if the amplitude of the rarefactional
pressure exceeds a certain threshold, which in turn
depends on ultrasound frequency with lower frequencies
having lower rarefactional pressure thresholds. Cavitation
threshold has been measured in different tissues in a number
of studies, but there is still no agreement ( 21-23, 28). For
example, cavitation threshold in blood is estimated to be 6.5
MPa ( 23) at 1.2 MHz. Once formed, the bubble can interact with the incident
ultrasound wave in two ways: stably or inertially. When the
bubble is exposed to a low-amplitude ultrasound field, the
oscillation of its size follows the pressure changes in the
sound wave and the bubble remains spherical. Bubbles that
have a resonant size with respect to the acoustic wavelength
will be driven into oscillation much more efficiently than
others; for ultrasound frequencies commonly used in HIFU
the resonant bubble diameter range is 1-5 microns ( 24).
Inertial cavitation is a more violent phenomenon, in which
the bubble grows during the rarefaction phase and then
rapidly collapses which leads to its destruction. The collapse
is often accompanied by the loss of bubble sphericity and
formation of high velocity liquid jets. If the bubble collapse
occurs next to a cell, the jets may be powerful enough to
cause disruption of the cell membrane ( 25, 26). In blood vessels, violently collapsing bubbles can damage
the lining of the vessel wall or even disrupt the vessel
altogether. One may assume that the disruption occurs due
to bubble growth and corresponding distension of the vessel
wall. However, it was shown that most damage occurs as the
bubble rapidly collapses and the vessel wall is bent inward
or invaginated, causing high amplitude shear stress ( 27). Stable cavitation may lead to a phenomenon called
“microstreaming” (rapid movement of fluid near the bubble
due to its oscillating motion). Microstreaming can produce
high shear forces close to the bubble that can disrupt cell
membranes and may play a role in ultrasound-enhanced
drug or gene delivery when damage to the cell membrane is
transient ( 28). Cavitation activity is the major mechanism that is
utilized when mechanical damage to tissue is a goal. At its
extreme, when very high rarefactional pressures (> 20 MPa)
are used, a cloud of cavitating bubbles can cause complete
tissue lysis at the focus ( 29). In such treatments the thermal
effect is usually to be avoided, therefore, short bursts of very
high amplitude ultrasound of low frequency (usually below
2 MHz) are used. The time-averaged intensity remains low,
and the thermal dose delivered to the tissue is not sufficient
to cause thermal damage. Cavitation can also promote heating if longer HIFU pulses or continuous ultrasound
is used ( 30-32). The energy of the incident ultrasound
wave is transferred very efficiently into stable oscillation
of resonant-size bubbles. This oscillatory motion causes
microstreaming around the bubbles and that, in turn, leads
to additional tissue heating through viscous friction, which
can lead to coagulative necrosis.
Nonlinear ultrasound propagation effects
Nonlinear effects of ultrasound propagation are observed
at high acoustic intensities and manifest themselves as
distortion of the pressure waveform: a sinusoidal wave
initially generated by an ultrasound transducer becomes
sawtooth-shaped as it propagates through water or tissue
( Figure 2D). This distortion represents the conversion of
energy contained in the fundamental frequency to higher
harmonics that are more rapidly absorbed in tissue since
ultrasound absorption coefficient increases with frequency.
As a result, tissue is heated much faster than it would if
nonlinear effects did not occur. Therefore, it is critical to
account for nonlinear effects when estimating a thermal
dose that a certain HIFU exposure would deliver. For most
clinically relevant HIFU transducers, nonlinear effects start
to be noticeable if the intensity exceeds 4000 W/cm 2, and at
9000 W/cm 2 it dominates over linear propagation ( 33). Probably, the most important consequence of nonlinear
propagation effects is that the boiling temperature of water,
100oC, can be achieved as rapidly as several milliseconds,
which leads to the formation of a millimeter-sized boiling
bubble at the focus of the transducer ( 34). This changes the
course of treatment dramatically: the incident ultrasound
wave is now reflected from the bubble and heat deposition
pattern is distorted in unpredictable manner. The lesion
shape becomes irregular, generally resembling a tadpole, as
illustrated in Figure 3B. Moreover, the motion of the boiling
bubble may cause tissue lysis that can be seen as a vaporized
cavity in the middle of the thermal lesion. Sometimes
this effect may be desirable and can be enhanced by using
HIFU pulses powerful enough to induce boiling in several
milliseconds, and with duration only slightly exceeding
the time to reach boiling temperature ( 35). In that case the
temperature rise is too rapid for protein denaturation to
occur, but the interaction of the large boiling bubble with
ultrasound field leads to complete tissue lysis, as illustrated
in Figure 3C ( 36).
Radiation force and streaming
Radiation force is exerted on an object when a wave is either
absorbed or reflected from that object. Complete reflection
produces twice the force that complete absorption does.
In both cases the force acts in direction of ultrasound propagation and is constant if the amplitude of a wave is
steady. If the ref lecting or absorbing medium is tissue or
other solid material, the force presses against the medium,
producing a pressure termed “radiation pressure.” For most
clinically relevant devices and exposures this effect is not
very pronounced: radiation pressure does not exceed a few
pascals ( 14). However, if the medium is liquid (i.e., blood)
and can move under pressure, then such pressure can induce
streaming with speeds of up to 6 m/s ( 37). This effect has
important implications in sonotrombolysis, in which a clotdissolving
agent is driven by streaming towards and inside
the clot blocking a vessel ( 38).
Image guidance and monitoring of HIFU therapy
There are currently two imaging methods employed in
commercially available HIFU devices: magnetic resonance
imaging (MRI) and diagnostic ultrasound. The role of
these methods in treatment is three-fold: visualization of
the target, monitoring tissue changes during treatment and
assesment of the treatment outcome. In terms of tumor
visualization, both MRI and sonography can provide
satisfactory images; MRI is sometimes superior in obese
patients ( 39), but is more expensive and labor-intensive. Unfortunately, to date none of the monitoring methods
can provide the image of the thermal lesion directly and in
real time as it forms in tissue. The biggest advantage of MRI
is that, unlike ultrasound-based methods, it can provide
tissue temperature maps overlying the MR image of the
target almost in real time. The distribution of sufficient thermal dose is then calculated and assumed to correspond
to thermally ablated tissue. The temporal resolution of
MR thermometry is 1-4 seconds per image, and the spatial
resolution is determined by the size of the image voxel
which is typically about 2mm x 2mm x 6mm ( 40). Therefore,
MR-guided HIFU is only suitable for treatments in which
the heating occurs slowly, on the order of tens of seconds
for a single lesion. Motion artifact due to breathing and
heartbeat is also a concern in clinical setting. The only US
FDA-approved HIFU device available for clinical therapy
utilizes MR thermometry during treatment of uterine
fibroids ( 39, 41). Ultrasound imaging used in current clinical devices does
not have the capability of performing thermometry, but it
provides real-time imaging using the same energy modality
as HIFU. This is a significant benefit, because adequate
ultrasound imaging of the target suggests that there is no
obstruction (e.g., bowel gas or bone) to ultrasound energy
reaching the target, and the risk of causing thermal injury
to unintended tissue is minimized. One method that is
sometimes used for confirmation of general targeting
accuracy is the appearance of a hyperechoic region on the
ultrasound image during treatment. This region has been
shown to correspond to the formation of a large boiling
bubble at the focus when tissue temperature reaches 100oC,
and underestimates the actual size of the thermal lesion
since thermal lesions develop at temperatures below 100oC
( 42). Imaging methods to assess HIFU treatment are similar
to those used to assess the response to other methods
of ablation such as radiofrequency ablation and include
contrast enhanced CT and MRI ( 43). In addition, the use
of microbubble contrast-enhanced sonography is also being
examined as a method to evaluate the treatment effect
of HIFU ( 44). These methods all examine the change in
vascularity of the treated volume.
|
|
HIFU of pancreatic tumors
Devices
Currently, HIFU treatment of pancreatic cancer is widely
available in China, with limited availability in South Korea
and Europe. There are two US-guided HIFU devices that
are commercially available outside of China for treatment
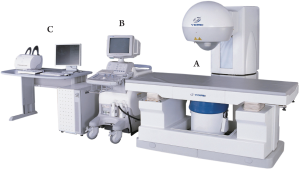
of pancreatic tumors, both manufactured in China: The
FEP-BY™ HIFU tumor therapy device (Yuande Biomedical
Engineering Limited Corporation, Beijing, China, Figure 4)
and HAIFU (Chongqing Haifu Technology Co.,) ( 45). Both
devices operate at similar ultrasound frequencies – 0.8 and
1 MHz respectively; both are capable of putting out total
acoustic power of about 300 W (corresponding intensity up to 20 000W/cm 2). B-mode ultrasound is also used in both
machines for targeting and image guidance. In addition, a
patient with pancreatic tumor was recently treated in Italy
using the MR-guided ExAblate™ system (InSightec, Israel)
for palliation of pain.
Animal studies
All the preclinical in vivo studies of HIFU ablation of
the pancreas utilized the swine model because of its size
and anatomy relevance to humans ( 46-48). The animals
were not bearing tumors in the pancreas, therefore, it
was not possible to evaluate survival benefits of HIFU
therapy; however, the main goal of these studies was
to systematically evaluate the safety and efficacy of
HIFU ablation of the pancreas. In the earliest study
the pancreata of 12 common swine were successfully
treated in vivo using the FEP-BY02 device, without
any significant adverse effects such as skin burns or
evidence for pancreatitis during the 7-day post-treatment
observation period ( 46). A subsequent study by another
group ut i l izing the HAIFU dev ice used both l ight
microscopy and electron microscopy to confirm that
complete necrosis is confined to the target regions with
clear boundaries and no damage to adjacent tissues ( 47).
Pancreatitis was an important safety concern because the
mechanical effects of HIFU can cause cell lysis and release
of pancreatic enzymes. Although the cavitation or boiling
bubble activity during HIFU was confirmed by electron
microscopic examination (intercellular space widening
and numerous vacuoles of different sizes in the cytoplasm),
pancreatitis was not observed thus confirming the safety
of treatment protocol. Another preclinical study showed
that a combined treatment of HIFU ablation followed by
radiation therapy may be a promising method. The injury
to the targeted pancreas was increased compared to either modality alone, without additional injury outside of the
targeted region ( 48).
Clinical studies
As mentioned above, most patients diagnosed with
pancreatic cancer are considered inoperable and systemic
chemotherapy has only modest effect. Development of
effective local therapies and strategies for pain relief are
both important aspect of managing these patients. HIFU
has been first used for the palliative treatment of pancreatic
cancer in an open-label study in China in 251 patients with
advanced pancreatic cancer (TNM stages II–IV) ( 49).
HIFU therapy resulted in significant pain relief in 84% of
the patients. In some cases significant reduction of tumor
volume was achieved without any significant adverse effects
or pancreatitis, which appears to have prolonged survival.
Multiple nonrandomized studies that followed, mostly from
China, provided additional evidence to show that HIFU
does provide palliation of tumor-related pain and does not
cause adverse effects ( 12-14, 50-56). The mechanism of pain
relief in these patients is still unclear, but is hypothesized
to result from thermal damage to the nerve fibers in the
tumor. In two studies HIFU was used in combination
with systemic chemotherapy (gemcitabine), and similar
findings were reported in terms of pain relief and safety,
even suggesting a survival benefit ( 14, 51). Figure 5 shows
representative CT images of a pancreatic tumor before and
after HIFU therapy. In a small study from Europe ( 55) 6 patients with
pancreatic tumors in difficult locations were treated with
HIFU, the difficult location being defined as a tumor
adjacent to major blood vessels, gallbladder and bile ducts,
bowel, or stomach. This study was performed under general
anesthesia, after 3-days of bowel preparation to avoid the
presence of bowel gas in the acoustic pathway. Symptoms were clearly palliated within 24 hours after treatment in
all patients, and the amylase level showed no statistically
significant elevation over baseline 3 days after treatment.
According to PET/CT and MDCT scans, the entire tumor
volume was successfully ablated in all cases. A major
complication – portal vein thrombosis – was observed in
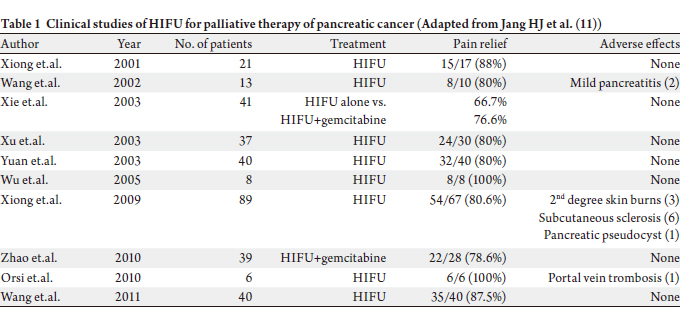
one patient, who was hospitalized for 7 days. The results of the studies are summarized in Table 1, and,
as seen, pain relief was achieved consistently in all studies.
However, no randomized, controlled trials have been
performed to date to confirm these findings or to determine
if HIFU can improve overall survival by inducing local tumor response.
|
|
Challenges and future directions
The major factors that complicate HIFU ablation of
pancreatic tumors are the presence of bowel gas, respiratory
motion and the absence of ultrasound-based temperature
monitoring methods. Bowel gas may obstruct the acoustic
window for transmission of HIFU energy, which may lead to
not only incomplete ablation of the target, but also thermal
damage to the bowel or colon due to rapid heat deposition
at the gas-tissue interface. Therefore, it is critical to evacuate the gas in the stomach and colon, which can be achieved by
having the patient fast the night before treatment. Applying
slight abdominal pressure to the target area also helps to
displace gas and clear the acoustic window.
Respiratory motion of the tumor during the treatment
leads to redistribution of acoustic energy over the area larger
than the focal region and may result in incomplete treatment
of the target and damage to adjacent tissues. Respiratory
motion tracking techniques that would allow for rapid focal
adjustment in sync with the target position are currently in
development ( 57). An approach that would avoid both the
problem of bowel gas and respiratory motion altogether is
the use of a miniature HIFU transducer integrated with
an endoscopic ultrasound probe. This approach would be
particularly beneficial in obese patients. Such miniature
endoscopic systems are not yet available commercially, but
are currently in development. Another problem that is inherent to any HIFU system
with ultrasound guidance is the absence of direct operator
control over the thermal dose that the target tissue received.
In order to estimate thermal dose, one needs to know
the output acoustic energy of the device, the absorption
coefficient of the target tissue and the attenuation by the
intervening tissue (primarily abdominal wall and viscera).
Therefore, careful calibration of HIFU fields and studies on
in-vivo measurement of acoustic attenuation and absorption
in different tissues are of great importance ( 46). |
|
Summary
HIFU ablation has been shown a promising method for
palliative treatment of pancreatic tumors. A number of
preliminary studies suggest that this technique is safe
and can be used alone or in combination with systemic
chemotherapy or radiation therapy. Further clinical trials
are currently being planned and will help to define the
future role of HIFU in the treatment of patients with
pancreas cancer.
|
|
References
- National Cancer Institute. Estimated New Cancer Cases and Deaths for
2009. Available online: http://seer.cancer.gov/csr/1975_2006.[LinkOut]
- Nakakura EK, Yeo CJ. Periampullary and pancreatic cancer. In:
Blumgart LH, ed. Surgery of the Liver, Biliary Tract, and Pancreas.4th
ed. Philadelphia: Saunders; 2007:849–57.
- Faivre J, Forman D, Estève J, Obradovic M, Sant M. Survival of patients
with primary liver cancer, pancreatic cancer and biliary tract cancer in
Europe. EUROCARE Working Group. Eur J Cancer 1998;34:2184-90.[LinkOut]
- Reddy SK, Elsayem A, Talukdar R. Supportive Care: Symptom
Management. In: Von Hoff DD, Evans DB, Hruban RH, eds. Pancreatic Cancer. Sudbury: Jones and Bartlett; 2005, pp 479-98.
- Cherny N, Ripamonti C, Pereira J, Davis C, Fallon M, McQuay H, et al.
Strategies to manage the adverse effects of oral morphine: an evidencebased
report. J Clin Oncol 2001;19:2542-54.[LinkOut]
- Yan BM, Myers RP. Neurolytic celiac plexus block for pain control in
unresectable pancreatic cancer. Am J Gastroenterol 2007;102:430-8.[LinkOut]
- Wu F, Wang ZB, Chen WZ, Zou JZ, Bai J, Zhu H, et al. Advanced
hepatocellular carcinoma: treatment with high-intensity focused
ultrasound ablation combined with transcatheter arterial embolization.
Radiology 2005;235:659–67.[LinkOut]
- Aus G. Current status of HIFU and cryotherapy in prostate cancer--a
review. Eur Urol 2006; 50:927–34;discussion 934.[LinkOut]
- Ren XL, Zhou XD, Zhang J, He GB, Han ZH, Zheng MJ, et al.
Extracorporeal ablation of uterine fibroids with high-intensity focused
ultrasound: imaging and histopathologic evaluation. J Ultrasound Med
2007;26:201–12.[LinkOut]
- Dubinsky TJ, Cuevas C, Dighe MK, Koloky thas O, Hwang JH.
High-intensity focused ultrasound: current potential and oncologic
applications. AJR Am J Roentgenol 2008;190:191-9.[LinkOut]
- Jang HJ, Lee JY, Lee DH, Kim WH, Hwang JH. Current and Future
Clinical Applications of High-Intensity Focused Ultrasound (HIFU)
for Pancreatic Cancer. Gut and Liver 2010;4:s57-61.[LinkOut]
- Wu F, Wang ZB, Zhu H, Chen WZ, Zou JZ, Bai J, et al. Feasibility of
US-guided high-intensity focused ultrasound treatment in patients
with advanced pancreatic cancer: initial experience. Radiolog y
2005;236:1034-40.[LinkOut]
- Xiong LL, Hwang JH, Huang XB, Yao SS, He CJ, Ge XH, et al. Early
clinical experience using high intensity focused ultrasound for
palliation of inoperable pancreatic cancer. JOP 2009;10:123-9.[LinkOut]
- Zhao H, Yang G, Wang D, Yu X, Zhang Y, Zhu J, et al. Concurrent
gemcitabine and high-intensit y focused ultrasound therapy in
patients with locally advanced pancreatic cancer. Anticancer Drugs
2010;21:447-52.[LinkOut]
- Lele P, Pierce A. The thermal hypothesis of the mechanism of ultrasonic
focal destruction in organized tissues. Proc. Workshop on Interaction
of Ultrasound and Biological Tissues 1972; 73-8008:121.
- Hill CR, Bamber JC, ter Haar GR. Physical Principles of Medical
Ultrasonics, 2nd edition. West Sussex,UK: John Wiley & Sons;2004.[LinkOut]
- Sapareto SA, Dewey WC. Thermal dose determination in cancer
therapy. Int J Radiat Oncol Biol Phys 1984;10:787-800.[LinkOut]
- ONeil HT. Theory of Focusing Radiators . J Acous t Soc Am
1949;21:516-26.[LinkOut]
- Vykhodtseva N, McDannold N, Martin H, Bronson RT, Hynynen K.
Apoptosis in ultrasound-produced threshold lesions in the rabbit brain.
Ultrasound Med Biol 2001;27:111–7.[LinkOut]
- Fujitomi Y, Kashima K, Ueda S, Yamada Y, MoriH, Uchida Y.
Histopathological features of liver damage induced by laser ablation in
rabbits. Lasers Surg Med 1999;24:14–23.[LinkOut]
- Apfel RE, Holland CK. Gauging the likelihood of cavitation from shortpulse,
low-duty cycle diagnostic ultrasound. Ultrasound Med Biol
1991;17:179–85.[LinkOut]
- Church CC. Spontaneous homogeneous nucleation, inertial cavitation
and the safety of diagnostic ultrasound. Ultrasound Med Biol
2002;28:1349-64.[LinkOut]
- Hwang JH, Tu J, Brayman AA, Matula TJ, Crum LA. Correlation
between inertial cavitation dose and endothelial cell damage in vivo.
Ultrasound Med Biol 2006;32:1611-9.[LinkOut]
- Bailey MR, Khokhlova VA, Sapozhnikov OA, Kargl SG, Crum LA.
Physical Mechanisms of the Therapeutic Effect of Ultrasound (A
Review). Acoustical Physics 2003;49:369–88.[LinkOut]
- Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis
by single oscillating bubbles. Nature 2003;423:153–6.[LinkOut]
- Schlicher RK, Hutcheson JD, Radhakrishna H, Apkarian RP, Prausnitz
MR. Changes in cell morphology due to plasma membrane wounding
by acoustic cavitation. Ultrasound Med. Biol 2010;36:677-92.[LinkOut]
- Chen H, Kreider W, Brayman AA, Bailey MR, Matula TJ. Blood vessel
deformations on microsecond time scales by ultrasonic cavitation. Phys
Rev Lett 2011;106:034301.[LinkOut]
- Holland CK, Apfel RE. Thresholds for transient cavitation produced by
pulsed ultrasound in a controlled nuclei environment. J Acoust Soc Am
1990;88:2059–69.[LinkOut]
- Parsons JE, Cain CA, Abrams GD, Fowlkes JB. Pulsed cavitational
ultrasound therapy for controlled tissue homogenization. Ultrasound
Med Biol 2006;32:115–29.[LinkOut]
- Sokka SD, King R, Hynynen K. MRI-guided gas bubble enhanced
ultrasound heating in in vivo rabbit thigh. Phys Med Biol 2003;48:223–
41.[LinkOut]
- Melodelima D, Chapelon JY, Theillère Y, Cathignol D. Combination
of thermal and cavitation effects to generate deep lesions with an
endocavitary applicator using a plane transducer: ex vivo studies.
Ultrasound Med Biol 2004;30:103–11.[LinkOut]
- Holt RG, Roy RA. Measurements of bubble-enhanced heating from
focused, MHz-frequency ultrasound in a tissue-mimicking material.
Ultrasound Med Biol 2001;27:1399–412.[LinkOut]
- Canney MS, Bailey MR, Crum LA, Khokhlova VA, Sapozhnikov OA.
Acoustic characterization of high intensity focused ultrasound fields:
a combined measurement and modeling approach. J Acoust Soc Am
2008;124:2406–20.[LinkOut]
- Canney MS, Khokhlova VA, Bessonova OV, Bailey MR, Crum LA.
Shock-induced heating and millisecond boiling in gels and tissue due to
high intensity focused ultrasound. Ultrasound Med Biol 2010;36:250–
67.[LinkOut]
- Canney M, Khokhlova V, Hwang JH, Khokhlova T, Bailey M, Crum
L. Tissue erosion using shock wave heating and millisecond boiling in
high intensity ultrasound field. Proc. 9th International Symposium on
Therapeutic Ultrasound 2009;p36-9.
- Khokhlova TD, Canney MS, VA Khokhlova, OA Sapozhnikov, LA
Crum, MR Bailey. Controlled tissue emulsification produced by high
intensity focused ultrasound shock waves and millisecond boiling. J
Acoust Soc Am 2011 (in press).[LinkOut]
- Mark F. Hamilton, David T. Blackstock. Nonlinear Acoustics. London:
Academic Press; 1998.
- Atar S, Luo H, Nagai T, Siegel RJ. Ultrasonic thrombolysis: catheterdel
ivered and transcutaneous applications. Eur J Ult rasound
1999;9:39-54.[LinkOut]
- Yagel S. High-intensity focused ultrasound: a revolution in non-invasive
ultrasound treatment? Ultrasound Obstet Gynecol 2004;23:216–7.[LinkOut]
- Köhler MO, Denis de Senneville B, Quesson B, Moonen CT, Ries M.
Spectrally selective pencil-beam navigator for motion compensation
of MR-guided high-intensity focused ultrasound therapy of abdominal
organs. Magn Reson Med 2011;66:102-11.[LinkOut]
- Gorny KR, Woodrum DA, Brown DL, Henrichsen TL, Weaver AL,
Amrami KK, et al. Magnetic resonance-guided focused ultrasound
of uterine leiomyomas: review of a 12-month outcome of 130 clinical
patients. J Vasc Interv Radiol 2011;22:857-64.[LinkOut]
- Khokhlova VA, Bailey MR, Reed JA, Cunitz BW, Kaczkowski PJ, Crum
LA. Effects of nonlinear propagation, cavitation, and boiling in lesion
formation by high intensity focused ultrasound in a gel phantom. J
Acoust Soc Am 2006;119:1834-48.[LinkOut]
- Jolesz FA, Hynynen K, McDannold N, Tempany C. MR imagingcontrolled
focused ultrasound ablation: a noninvasive image-guided
surgery. Magn Reson Imaging Clin N Am 2005;13:545–60.[LinkOut]
- Kennedy JE, ter Haar GR, Wu F, Gleeson FV, Roberts IS, Middleton
MR, et al. Contrast-enhanced ultrasound assessment of tissue
response to high-intensity focused ultrasound. Ultrasound Med Biol
2004;30:851-4.[LinkOut]
- Haar GT, Coussios C. High intensity focused ultrasound: physical
principles and devices. Int J Hyperthermia 2007;23:89-104.[LinkOut]
- Hwang JH, Wang YN, Warren C, Upton MP, Starr F, Zhou Y, et al.
Preclinical in vivo evaluation of an extracorporeal HIFU device for
ablation of pancreatic tumors. Ultrasound Med Biol 2009;35:967-75.[LinkOut]
- Xie B, Li YY, Jia L, Nie YQ, Du H, Jiang SM. Experimental ablation
of the pancreas with high intensity focused ultrasound (HIFU) in a
porcine model. Int J Med Sci 2010;8:9-15.[LinkOut]
- Liu CX, Gao XS, Xiong LL, Ge HY, He XY, Li T, et al. A preclinical in
vivo investigation of high-intensity focused ultrasound combined with
radiotherapy. Ultrasound Med Biol 2011;37:69-77.[LinkOut]
- He SX, Wang GM. The noninvasive treatment of 251 cases of advanced
pancreatic cancer with focused ultrasound surger y. Proc. 2nd
International Symposium on Therapeutic Ultrasound 2002;p51-56.
- Wang X, Sun JZ. Preliminary study of high intensity focused ultrasound
in treating patients with advanced pancreatic carcinoma. Chin J Gen
Surg 2002; 17:654-5.
- Xie DR, Chen D, Teng H. A multicenter non-randomized clinical study
of high intensity focused ultrasound in treating patients with local
advanced pancreatic carcinoma. Chin J Clin Oncol 2003;30:630-4.
- Xiong LL, He CJ, Yao SS, Zeng JQ, Zhang GX, Huang K, et al. The
preliminary clinical results of the treatment for advanced pancreatic
carcinoma by high intensity focused ultrasound. Chin J Gen Surg
2005;16:345-7.
- Xu YQ, Wang GM, Gu YZ, Zhang HF. The acesodyne effect of high
intensity focused ultrasound on the treatment of advanced pancreatic
carcinoma. Clin Med J China 2003;10:322-3.
- Yuan C, Yang L, Yao C. Obser vation of high intensit y focused
ultrasound treating 40 cases of pancreatic cancer. Chin J Clin Hep
2003;19:145.
- Orsi F, Zhang L, Arnone P, Orgera G, Bonomo G, Vigna PD, et al. Highintensity
focused ultrasound ablation: effective and safe therapy for
solid tumors in difficult locations. AJR Am J Roentgenol 2010;195:
W245-52.[LinkOut]
- Wang K, Chen Z, Meng Z, Lin J, Zhou Z, Wang P, et al. Analgesic effect
of high intensity focused ultrasound therapy for unresectable pancreatic
cancer. Int J Hyperthermia 2011;27:101-7.[LinkOut]
- Muratore R, Lizzi FL, Ketterling JA, Kalisz A, Bernardi RB, Vecchio
CJ. A System Integrating HIFU Exposure Capabilities with Multiple
Modes of Synchronous Ultrasonic Monitoring. Proc.4th International
Symposium on Therapeutic Ultrasound 2005:pp33-5.
|