Can pancreaticoduodenectomy performed at a comprehensive
community cancer center have comparable results as major
tertiary center?
Appleton Medical Center, Fox Valley Surgical Associates, Appleton, Wisconsin, USA
|
Original Article
Can pancreaticoduodenectomy performed at a comprehensive
community cancer center have comparable results as major
tertiary center?
Appleton Medical Center, Fox Valley Surgical Associates, Appleton, Wisconsin, USA
|
|
Abstract
Background: Pancreatic resection is a definitive treatment modality for pancreatic neoplasm. Pancreaticoduodenectomy
(PD) is the primary procedure for tumor arising from head of pancreas. Prognosis is overwhelmingly poor despite
adequate resection. We maintained a prospective database covering years 2001 to 2010. Outcome data is analyzed and
compared with those from tertiary centers.
Methods: Sixty-two patients with various histology were included. Pylorus preserving pancreatico-duodenectomy
(PPPD), classic pancreaticoduodenectomy, and subtotal pancreatectomy were procedures performed. Three patients had
portal venorrhaphy performed to obtain clinically negative margin. Forty six patients had malignancy on final pathologic
analysis.
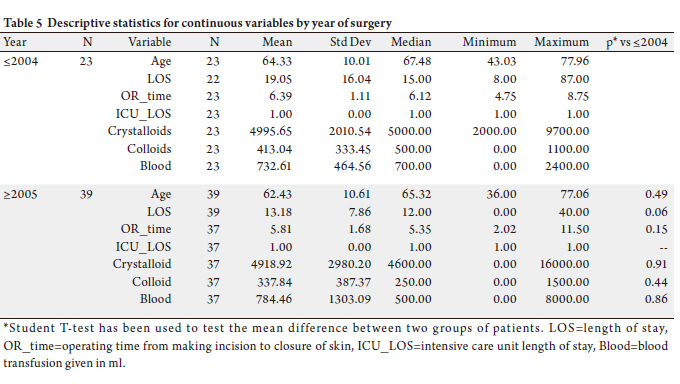
Results: The average age of patients was 63. Mean preoperative CA19-9 for exocrine pancreatic malignancies was
higher than for more benign lesions. There was a decrease in operative time during this period. Blood transfusion was
uncommon. There was very few pancreatic leak among the patients. Two bile leaks were identified, one controlled with
the drainage tube and the other one required repeat surgery. The primary reason for the prolonged hospitalization was
gastric ileus. For patients without a gastrostomy tube, nasogastric tube was kept in until gastric ileus resolved. 30 days
mortality rate was calculated at 4.8. Mean survival time during our follow up was 30.6 months. Comparing to published
literature, present series’ mortality, morbidity, and survival are similar. Five year survival was 39%.
Conclusion: Despite overall poor outcome for patients with pancreatic and biliary malignancies, we conclude that
surgery can be performed in community hospitals with special interest in treating pancreatic disorder, offering patients
equivalent survival and quality of life as those operated in tertiary centers.
Key words
Pancreaticoduodenectomy for pancreatic cancer; surgical outcome; community hospital
J Gastrointest Oncol 2011; 2: 143-150. DOI: 10.3978/j.issn.2078-6891.2011.035
|
|
Introduction
With about 44000 new cases and about 37600 cancer deaths
in 2011, pancreatic cancer ranks fourth among cancerrelated
deaths in the United States. It is the second leading cause of death due to gastrointestinal tract neoplasm. It is
one of the few cancers whose survival has not improved over
the past 40 years (1).
Pancreatic cancer affects more commonly elderly, and
less than 20% of patients present with localized, potentially
curable tumors (2). The average life expectancy after
diagnosis with metastatic disease is three to six months.
Average five year survival is 6%. Seventy-five percent of
patients die within first year of diagnosis. Pancreatic cancer
has the highest death rate of all major cancers (3).
Symptoms of pancreatic cancer depend on the location,
as well as on the stage of the disease. Significant number
of tumors develops in the head of the pancreas and
usually led to cholestasis, abdominal discomfort and
nausea. Obstruction of the pancreatic duct may lead to pancreatitis. Most patients have systemic manifestations of
the disease such as asthenia, anorexia, and weight loss. Less
common manifestations include venous thrombosis, liverdysfunction,
gastric obstruction, and depression (4-6).
Pancreaticoduodectomy (PD) is the most commonly
performed surgery in patients with pancreatic cancer as 75%
of tumors are located at head of pancreas. First successful
pancreatic head resection was described by Walter Kausch
in 1912, and later modified by Allen O Whipple in 1935 as
two stage procedure whereby diversion was followed by
definitive resection (7,8).
|
|
Method
In Appleton, Wisconsin, a community hospital cancer
center was established in 2001. Patients underwent PD
were followed from 2001 to 2010, 62 PD’s were performed
during this time interval by a surgical team with interest in
gastrointestinal oncology. The results were compared with
a large series of similar surgery performed elsewhere in the
United States (9). The retrospective analysis of the database
was approved by the local Institutional Review Board of
ThedaCare Hospitals.
SAS 9.2 statistical sof tware was used to perform
statistical analysis. Student t-test was used to test the mean
difference between two groups of patients. Fisher’s exact
test was used to examine the association between two
factors in a table. Kaplan Meier survival curves were used to
estimate survival.
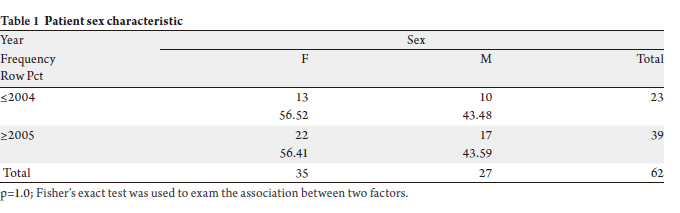
A total of 62 patients (female 35, male 27) with histologyproven
pancreatic cancer, ampullary carcinoma and other
histological types, including benign histological entities,
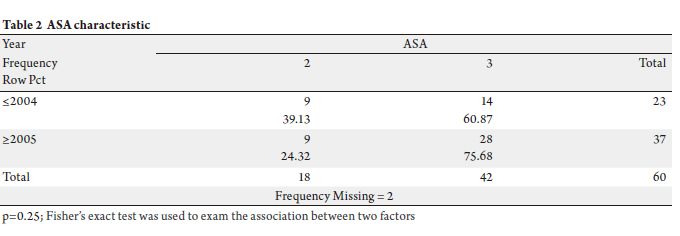
were included in the study (Tables 1 & 2). To query on the
difference in outcome between the early and later time
interval, we arbitrarily analyzed patients operated before
and after year 2005.
Pylorus preserving pancreaticoduodenectomy
(PPPD) was performed in forty one patients; twenty
patients had traditional PD and one patient with subtotal
pancreatectomy. Clinical pathway was adapted and utilized
uniformly in the later period. Three patients had portal
venorrhaphy due to tumor adherence to the portal vein.
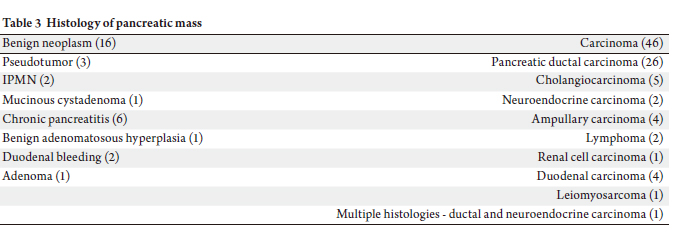
Forty six patients had malignant diagnoses, whereas sixteen
patients had benign histology. One case had dual histology
(ductal carcinoma and neuroendocrine tumor).
Final pathology showed pancreatic adenocarcinoma,
cholangiocarcinoma, adenoma, lymphoma, ampullary
carcinoma, duodenum carcinoma, leiomyosarcom, isolated
metastatic carcinoma to pancreas, and neuroendocrine
tumor. Benign histological diagnoses included,
pancreatitis, IPMN, pseudotumor, and adenomatous hyperplasia (Table 3).
Majority of patients presented with jaundice, weight
loss and abdominal pain. All of the patients had computed
tomography scan done as part of their evaluation.
Endoscopic retrograde cholangiopancreatography (ERCP)
was performed for patients with symptoms related to bile
duct obstruction. Preoperative biliary stents were placed
at the discretion of the endoscopist, with relief of jaundice
being the primary intent.
Mean age of patients was 63 years, with ages ranging
from 39 to 78 years. Ethnicity among the patients included
34 Caucasians, 3 Asians, 5 Hispanics, and 13 patients of
unknown origin.
   |
|
Clinical data
Average operative time was 385 minutes for surgeries
performed before 2005 and 348 minutes for surgeries
performed after 2005. Comparing procedures performed
pre-and post-2005, length of hospital stay was shorter
(nearly reaching statistical significance) adjusted for
gender, age, and ASA (p=0.06). Average length of stay for
all patients was 16.1 days (range 0-87 days), mean ICU
stay was 3 days (range 1-63 days). Among the covariates
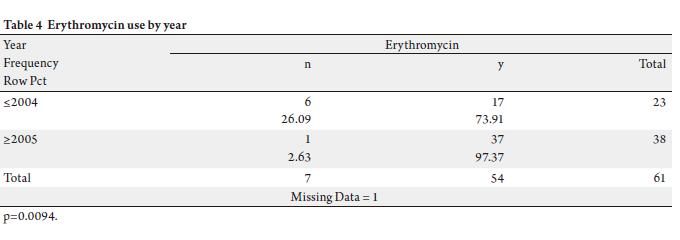
examined, only erythromycin use (as motility agent)
changed significantly: there was a substantial increase
in its usage (p=0.009). Erythromycin was ordered for 17
(73.91%) patients out of 23 surgeries performed before
2005 and 97.4% of patients received Erythromycin after the
surgery (Table 4).
Blood transfusion was given to 15 patients requiring
blood product. Mean preoperative CA19-9 for exocrine
pancreatic malignancies was 638, whereas for benign lesions
and endocrine tumors it was 122 (Table 5).
There were three perioperative deaths due to ischemic
bowel and severe acidosis, equivalent to thirty day mortality
rate of 4.8%. Major causes of 30 day postoperative death in
our study were small bowel necrosis (ii) and disseminated
intravascular coagulopathy (i). There was one pancreatic
leak in our patient population. Two bile leaks were
identified, one controlled with the drainage tube and one
required laparotomy to repair the leak. Average length
of stay was 15 days. The primary reason for prolonged
hospitalization was gastric ileus. For patients without a
gastrostomy tube, nasogastric tube was kept in until gastric
ileus resolved.
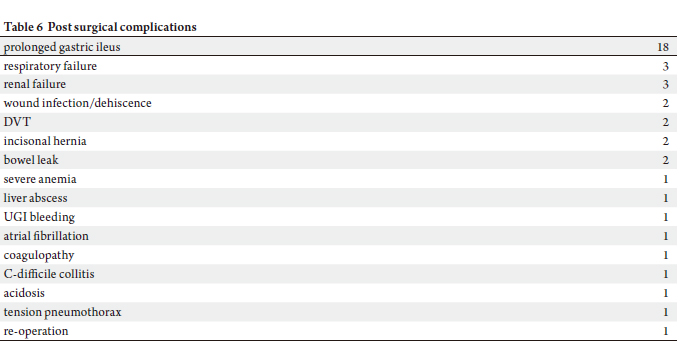
Respiratory failure and renal failure occurred in 4.8% of
patients. Wound infection, DVT, and incisional hernia each
comprises 3.2% of our patient population (Table 6).
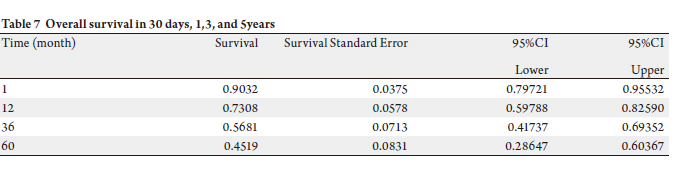
To date, 45% of our patients (N=28) have died, with
two patients from causes unrelated to carcinoma. Mean survival during our study period was 30.6 months for all 62
individuals (Tables 7 & 8). Three year survival for patients
with pancreatic cancer and carcinoma of non pancreas
origin were 39% and 66%, respectively.
In our series of patients, 47.9% had metastatic disease
in regional lymph nodes. 14.2% had positive margins.
For patients without lymph node metastasis and negative
margin, survival was 75%, 47%, and 47% at 12, 36 and
60 months post surger y, respectively. Patients with lymph node metastasis had 5 years survival rate of 39%
whereas those without lymph node involvement had
5 year survival of 48%. Majority of the patients were
of fered adjuvant chemoradiation therapy based on
tumor size greater than 2 cm or if lymph node metastasis
was present. Overall five year survival in this patient
population was 39% (Fig 1). Stage of cancer does not
appear to have an impact on survival. Stages I/II had
5 year survival of 36%, and stages III/IV patients had survival of 34% (Fig 2).
      |
|
Discussion
Our results were produced in a comprehensive community
cancer center accredited by the American College of
Surgeons Commission on Cancer. Multidisciplinary
discussions were held during regularly scheduled tumor
conferences. Many of the services providing diagnostic and
therapeutic work up are readily available within the medical
complex. Specialists with interest in gastrointestinal
oncology participate in discussion forums to formulate
treatment plans for each patient. Treatment progress notes
are made available shortly after each encounter with the
patient with an electronic medical record system.
There are numerous publications demonstrating an
improvement of outcome after PD in high volume medical
centers (10-13). Surgeon volume alone also significantly
decreases mortality for complex procedures (14). An
analysis of high volume centers has shown that there is
a significant variability in mortality (0.7% to 7.7%) and,
with other variables analyzed, demonstrates that the variability cannot be explained by hospital volume alone
(15). Surgeon experience is an important determinant of
overall morbidity. In the same study, it was concluded that
experienced surgeons (those who have performed more
than fifty PD) have equivalent results whether they are high
volume surgeons (some performing more than 20 PD per
year) or low volume surgeons (16).
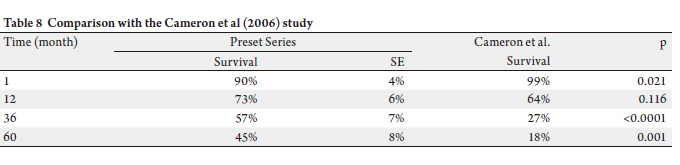
In the literature, five year survival for pancreatic cancer
patients treated with PD ranged from 3% in the early series
to 20% in more recent publications (16-18). In our series,
five year overall survival for patients treated for carcinoma
was 39% .
We have chosen a single institution series from Johns
Hopkins with one thousand consecutive PD to compare the
results between the two institutions. Mortality, morbidity,
and survivals are similar (19,20).
The learning curve in pancreatic surgery suggested that
after 60 PD’s, there are improved outcomes of estimated
blood loss, operative time, length of stay, and margin status
— factors which have been associated with overall outcome
(21). The results presented in this study are consistent with
the conclusions presented by published literature.
The benefits of regionalization of complex surgery were
demonstrated in a number of studies. Benefits of a high
volume center include a decrease in mortality and cost and
the ability to perform prospective randomized trials and to
provide surgical training (22,23).
One of the goals of this study is to determine if we
can provide excellent care to patients diagnosed with
periampullary tumors. The closest medical center with
pancreaticobiliary service to our center is approximately
90 miles. Given the choice for location of service, an
overwhelming majority of patients preferred not to travel
long distances. Having a pancreaticobiliary service in our
encatchment area serves to facilitate treatment as well as to
allow patient’s family members easier access to the treating
medical center.
There has been a dramatic improvement of surgical care
in treating periampullary tumors over the last two decades.
Anesthetic and perioperative care during the duration of
our study have made the greatest contribution to decreasing
perioperative mortality. The development of clinical
pathways also has contributed to optimizing the outcome
(24).
There are limitations to a single institutional series such
as ours. Patient population is not large. Because of the
small number of patients, meaningful statistical analysis
is difficult to derive. Morbidity, mortality, and long term
outcomes (cancer specific survival, overall survival)
nevertheless have utility in assessing a cancer program.
The data presented here gives support to continuing the
pancreaticobiliary program at our institution.
Our results ref lect the dedication of specialists with
interest in treating pancreaticobiliary disorders. We assert
that hospital volume alone cannot be the sole determinant
of outcome. It is our belief that surgeon volume combined
with a multidisciplinary approach and excellent ancillary
support provide an excellent prediction of survival as
demonstrated in this study of patients with pancreatic and
biliary malignancies.
The factors contributing to improved survival for patients
diagnosed with periampullary tumors are numerous.
Improved perioperative critical care and improved surgical
care decrease operating time. Advances in adjunctive
therapies contribute to improved survival. It is through
these novel therapies that we will see further improvement
in survival rates (25).
|
|
References
Cite this article as:
Cheng C, Duppler D, Jaremko B. Can pancreaticoduodenectomy performed at a comprehensive
community cancer center have comparable results as major
tertiary center? J Gastrointest Oncol. 2011;2(3):143-150. DOI:10.3978/j.issn.2078-6891.2011.035
|