The incidence of colorectal cancer in patients with previously removed polyp(s)—a cross-sectional study
Introduction
Because of the rising incidence of colorectal cancer and the presence of a clear precursor lesion, screenings programs have been introduced in order to detect cancer earlier with the expectation that survival will improve, and moreover development of cancer can be prevented. Already many years ago, it was established that removal of adenomas leads to a lower incidence of colorectal cancer in the years to follow (1).
There are several guidelines for follow-up (2). Data on the yield of the follow-up endoscopy with respect to development colorectal cancer are rather sparse. Mostly, studies are done in specific centers using selected groups of patients.
This study was done in unselected patients in normal daily practice, in whom at index colonoscopy polyp(s) were removed, in order to study the occurrence of colorectal cancer in the years to follow.
Methods
A prospectively collected dataset on colonoscopy covering 25 consecutive years was used. All patients underwent colonoscopy, for different clinical reasons, in the Zaans Medisch Centrum, the community hospital of the Zaanstreek region in The Netherlands.
Only patients in whom during the index procedure (this is the first procedure) a polyp(s) was detected and removed, were included. From each individual patient the data set was searched for further colonoscopies. The results of following endoscopies were noted.
Patients in whom during the index colonoscopy polyp(s) were seen synchronous with colorectal cancer and patients belonging to known Lynch families were excluded.
Also, all procedures done within 6 months after the index were excluded. These procedures were considered as immediate follow-up with inspection of prior polypectomy site(s) or further removal of earlier detected polyps.
In case of diagnosing colorectal cancer time after the index and previous procedure was noted. In these patients, tumor stage, histology of earlier removed polyps, localization of the tumor and demographics were noted.
Statistical analysis was done with Chi-square test for contingency tables and t-test. A value below 0.05 was considered significant.
Results
In the 25 years 34,531 procedures were done. In these procedures more than 9,356 cases (27%) were present in which polyps (hyperplastic, adenomatous, inflammatory, juvenile and others) were detected and removed. Only in 1,617 patients’ follow-up procedures, as defined in the methods section, were done. These patients underwent a total of 2,933 follow-up procedures. Many procedures were done in regular follow-up according to the guidelines. Patients also underwent new colonoscopy because of new or recurrent complaints.
In total, 30 (1.9%) of these patients developed colorectal cancer. Two patients (2 men, 62 and 9 months after the previous endoscopy) developed a new adenoma, judged to be benign during endoscopy. After removal and histological examination, the polyp appeared to be a small T1 tumor. One patient had four adenomas which could not be removed during the index endoscopy because of circulatory instability. This patient was lost to follow-up and returned 7 years later with cancer.
In 18 patients, adenomas were removed during previous endoscopies. Five patients only had hyperplastic polyp(s), no serrated adenomas. From seven patients, data could not be retrieved from the files. Nine patients in whom ultimately cancer was diagnosed were older than 75 years when the previous endoscopy was done. Cancer in these patients was detected because of analysis of abdominal complaints or anemia. Patients with adenomas prior to the cancer were older compared with patients with hyperplastic polyps [mean (SD): 71.6 (5.8) versus 64.2 (10.5) years, P=0.046]. There was no difference with patients whose data were missing (Table 1).

Full table
Table 2 shows the localization of the malignancies. The majority was located in the proximal colon (75%).
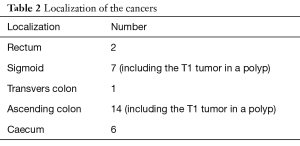
Full table
The patients in whom ultimately cancer was diagnosed underwent one follow-up colonoscopy in 15 cases; two follow-ups in seven, three in four, four in two, and one patient had five and another six follow-up procedures.
Table 3 shows the time between diagnosing cancer and the previous endoscopy. This was mean 70.6 months (SD, 40.8 months) with a median of 60.0 months (range, 12.0–167.0 months). In one patient, cancer in the sigmoid was diagnosed 12 months after removal of two adenomas in the sigmoid. This cancer, or the adenoma, was clearly missed. Excluding this patient did not dramatically change the above-mentioned result [mean, 72.7 months; SD, 39.6 months; median, 62.0 months (range, 24.0–167.0 months)].

Full table
Three patients did not undergo surgery (one because of metastatic disease and two because of major co-morbidity). One patient went to another hospital and was lost to follow-up. Table 4 shows the tumor stage. In Table 5 the number and yield of the follow-up colonoscopies is shown.
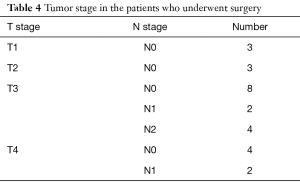
Full table

Full table
Discussion
Patients in whom adenomas are detected are offered participation in the regular follow-up program according to the applicable guidelines. Responsibility for the adherence to surveillance advice is left to the patient, family physician or gastroenterologist. Mulder et al. studied the yield of surveillance without active invitation to follow-up endoscopy.
They concluded that passive follow-up policies may lead to under-performance of surveillance programs (3). In the past 25 years these guidelines were updated several times (4,5). This is a possible explanation for the wide difference in time between follow-up procedures in the present study. In addition, not all patients actively participate depending on co-morbidity and life expectancy. Many patients are already older when the polyp(s) is detected. Doctors are not always compliant with guidelines. Despite all surveillance programs many patients do not attend. In a study by Atkin et al. fifty eight percent of patients did not attend surveillance. However, one or two surveillance visits were associated with a significant reduction in colorectal cancer incidence rate (adjusted hazard ratio: 0.57, 95% CI: 0.40–0.80 for one visit; adjusted hazard ratio: 0.51, 95% CI: 0.31–0.84 for two visits) (6). Reaching the age of 75 years generally is a reason to stop follow-up.
If hyperplastic polyps (not serrated adenomas) were detected in the index colonoscopy follow-up is not scheduled. However, these patients will undergo a next colonoscopy if there are clinical reasons to do so. This is the reason why ultimately cancer has been diagnosed in patients with prior hyperplastic polyps.
Are the cancers detected in the present study interval cancers? Interval cancer is defined as colorectal cancer appearing after a negative screening or surveillance test for colorectal cancer and before the recommended date of the following screening test. It has been estimated that up to 75% of interval colorectal cancers may be due to poor endoscopic technique (7). In fact, only one true interval cancer was seen in the present study, and this cancer or its precursor lesion was judged to be missed during previous endoscopy. The remaining cancers were diagnosed after a normal recommended surveillance time.
The study of Winawer et al. in 1993 showed a reduction of 94% of expected cancers in the national polyp study (1). The incidence of colorectal cancer in the present study was low (1.8%).
In The Netherlands, a very meticulous cancer registration exists (8). All cancers diagnosed are included in this system. It provides a risk analysis for people with respect to development of colorectal cancer. Although the follow-up time in the study population show a wide range, 0.5–23 years, this group can be used to calculate the life time risk for cancer. The mean follow-up was 11 years. Using the different age cohorts as given in the risk assessment, it was possible to calculate an estimate of the number of colorectal cancers that could have been expected. This is 25 rectal cancers and 52 colon cancers. Looking at the data in the present study only two rectal cancers and 28 colon cancers occurred. This is a reduction of 92% and 47% respectively. If patients with a hyperplastic polyp are excluded, this is because they did not have a reason for endoscopic follow-up; the reduction of colon cancers was higher, 56%.
Recent data report on a safe interval of 5 years for surveillance in case of low-risk adenomas (9). However, there is also evidence that small low risk adenomas have no need for follow-up (10). A possible flaw of the study is the fact that the data set did not report on the number of polyps, or the macroscopic or histological appearance.
The majority of cancer was located in the proximal colon. This may lead to the assumption that precursor lesions, this is serrated adenomas of flat adenomas, are missed during previous endoscopy. The most likely explanation is inadequate colon cleansing. Unfortunately, the data set did not include a score for the bowel preparation. This observation once again stipulates that excellent bowel preparation is essential.
The cancers were diagnosed approximately 8 years after an earlier procedure. However, there was a wide range, from 1 to 16.5 years. It is tempting to assume that most cancers will be detected within the normal recommended time of follow-up as given in the guidelines. Nine patients (53%) in whom adenoma(s) were removed prior to the diagnosis of cancer already reached an age that further follow-up is not recommended anymore.
It is concluded that follow-up after removal of adenomas in normal daily practice is associated with a decrease in development of colorectal cancer. However, to reach this many follow-up colonoscopies have to be done.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: For this kind of study ethical approval is not required in The Netherlands. In addition, not all patients are still alive, hence informed consent for using their data is impossible.
References
- Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med 1993;329:1977-81. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, Fletcher RH, et al. Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 2006;130:1872-85. [Crossref] [PubMed]
- Mulder SA, Van Leerdam ME, Ouwendijk RJ, et al. Attendance at surveillance endoscopy of patients with adenoma or colorectal cancer. Scand J Gastroenterol 2007;42:66-71. [Crossref] [PubMed]
- Winawer SJ, Zauber AG, O'Brien MJ, et al. Randomized comparison of surveillance intervals after colonoscopic removal of newly diagnosed adenomatous polyps. The National Polyp Study Workgroup. N Engl J Med 1993;328:901-6. [Crossref] [PubMed]
- Chung SJ, Kim YS, Yang SY, et al. Five-year risk for advanced colorectal neoplasia after initial colonoscopy according to the baseline risk stratification: a prospective study in 2452 asymptomatic Koreans. Gut 2011;60:1537-43. [Crossref] [PubMed]
- Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol 2017;18:823-34. [Crossref] [PubMed]
- Pellisé M. Colonoscopy in the screening, follow-up and treatment of colorectal cancer and precursor lesions. Gastroenterol Hepatol 2015;38 Suppl 1:71-7. [PubMed]
- . Available online: https://www.cijfersoverkanker.nl/Nederlandse Kankerregistratie.
- Anderson JC, Baron JA, Ahnen DJ, et al. Factors Associated With Shorter Colonoscopy Surveillance Intervals for Patients With Low-Risk Colorectal Adenomas and Effects on Outcome. Gastroenterology 2017;152:1933-43.e5. [Crossref] [PubMed]
- Jover R, Dekker E. Surveillance after colorectal polyp removal. Best Pract Res Clin Gastroenterol 2016;30:937-48. [Crossref] [PubMed]


