Combination immunotherapy and radiation therapy strategies for pancreatic cancer—targeting multiple steps in the cancer immunity cycle
Introduction
Current treatment strategies and investigations in pancreas cancer
Pancreatic ductal adenocarcinoma (PDAC), represents 3.2% of all new cancer cases in the United States annually, yet is responsible for approximately 7.2% of cancer-related deaths (1). The vast majority of patients are either locally advanced, unresectable, or metastatic at presentation (1), and even patients who are resectable have poor long-term survival outcomes relative to other early-stage malignancies (1). Commonly employed treatment for resectable patients includes surgical resection followed by chemotherapy or chemoradiotherapy (2). For locally advanced, unresectable patients, multi-agent chemotherapy alone, induction multi-agent chemotherapy followed by chemoradiotherapy, or chemoradiotherapy are all options for good performance status patients, though clinical trial is preferred (2), as results continue to be poor. The 5-year overall survival (OS) rate continues to be less than 10% for all stages (1), and given that pancreatic cancer is projected to become the second most common cause of cancer-related death by 2030 (3), much interest has been generated at both increasing the efficacy of current treatment modalities and investigating new treatment approaches.
One of these recently investigated approaches is targeted therapy. Unfortunately, erlotinib, a targeted agent to EGFR, when added to induction chemotherapy in unresectable patients, was associated with a trend to poorer survival in the most recent trial for locally advanced patents, LAP-07 (4). To further illustrate the difficulty of improving outcomes with targeted agents, it has been shown that while there are many targetable genes in pancreatic cancer, there were no dominant phenotypes. Most of the targetable mutations were found in only 1–7% of the patients in the sample (5). This makes it difficult to not only find potential treatment targets, but also to fully investigate them, both alone and in combination with other therapies.
Immuno-oncology in pancreas cancer
Given the poor results seen with the combination of chemotherapy, radiotherapy, surgery and even single targeted agents, as well as promising results in other cancers, much interest has been generated in the use of the immune system in the treatment of PDAC. This concept is known as immuno-oncology and can take two forms: a passive form that involves infusion of pre-formed immune products, such as antibodies and cytokines to directly target the tumor, and an active form that involves activating the patient’s immune system to attack tumor cells. Both active and passive forms are predicated on the idea that cancer cells express unique antigens that can be targeted by the immune system while potentially sparing normal tissues (6).
Immune checkpoint inhibitors
Clinical utilization of immune checkpoint inhibitors has been examined in adjuvant treatment for curable disease (7-9), first-line therapy for recurrent disease or initial metastatic failure (10,11), or second-line therapy in metastatic disease refractory to other treatment options (12,13). Much excitement has been generated by the responses to these checkpoint inhibitors in tumors that are refractory to traditional chemotherapy, as well as cancers that have developed resistance to traditional agents. Response rates (RR) in these situations can be anywhere from 20–60% (14), with the best responses seen in the most immunogenic tumors (i.e., melanoma). Unfortunately, the use of immune checkpoint inhibitors alone has encountered disappointing results in clinical trials in pancreas cancer, with a RR of only 1–2% in one study (15).
Although checkpoint inhibitors have been largely disappointing in pancreatic cancer, colon cancer, which is also often KRAS driven, has shown efficacy in combination with MEK1/2 inhibition. Pre-clinical modeling of murine mutant KRAS colon cancer demonstrated increased CD8+ T-cell tumor infiltration and cooperative tumor suppression/inhibition with PD-L1 (checkpoint) inhibitor with MEK1/2 inhibition (16). Initial clinical trials demonstrated combination of cobimetinib (MEK1/2 inhibitor) and atezolizumab (PD-L1 inhibitor) had a 9% RR (in KRAS mutated patients) and 67% survival at 6 months in patients who had failed previous lines of standard-of-care chemotherapy (17). Previously only micro-satellite stable colorectal cancer was shown to be responsive to checkpoint inhibition. Given that approximately 95% of pancreatic cancers harbor a KRAS mutation this may represent a viable combination for pancreatic cancer treatment as well.
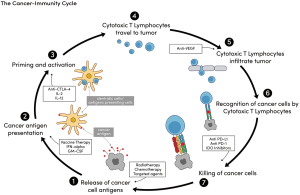
The cancer immunity cycle
It is increasingly being realized, however, that stimulating more than one aspect of the immune response may be needed to elicit an effect profound enough to improve clinical outcomes in many cancers that are less immunogenic, like pancreatic cancer. The cancer-immunity cycle is complicated and is believed to involve at least seven stages (Figure 1). An example of such multi-level involvement includes the reported “abscopal effect”, or the phenomenon in which localized treatment of one tumor results in some degree of regression in tumors distant from that site. This is the justification for the use of radiotherapy in conjunction with a systemic, immune-stimulatory agent, like IL-2. This approach targets both stage 1 (radiotherapy, tumor cell antigen release) and stage 3 (IL-2, priming and activation) of the cancer-immunity cycle (see Figure 1). Recent investigations suggest that the abscopal effect may depend on the immunogenicity of the tumor (18-21), thus in tumors like pancreatic cancer with low immunogenicity, multiple levels of immune activation may be required. In a study of long term [median survival (MS) >4 years] and short-term only (MS ~1 year) pancreatic cancer survivors, investigators used a computational algorithm to assign a neoantigen “quality score” for the resection specimen in each patient. Patients with both the highest neoantigen number and the most abundant CD8+ T-cell infiltrates experienced the longest survival (22). This suggests that there may be not only a role for immune modulation in these patients, but also that said modulation may require targeting multiple, as opposed to single, steps in the immune response.
This review will therefore focus on advances in both local and systemic agents that are thought to increase the adaptive immune response to pancreatic cancer. Open clinical trials assessing therapeutic approaches using combined radiation therapy (RT) and immune-stimulating agents will then be reviewed.
Methods
We reviewed potential immunotherapy strategies in pancreatic cancer both with and without RT. A PubMed and clinicaltrials.gov search was conducted using the following search terms, either alone or in combination: “pancreatic cancer”, “immunotherapy”, and “abscopal effect”. Twenty-one clinical trials were reviewed after narrowing down trials that involved pancreatic cancer and some form of immunotherapy, including vaccine therapy, immune checkpoint inhibition, or other non-traditional systemic therapy designed to prime the immune system. Some immunotherapy studies of cancers other than pancreatic were included for the sake of comparison. We compiled a table of selected open trials discussed in this review, as well as where they are open and estimated closure dates.
Results
Overcoming the immunosuppressive tumor microenvironment (TME)
One of the major challenges in the attempt to utilize the immune system in the treatment of PDAC is that these tumors tend to reside in an immunosuppressive TME. Part of that suppressive environment includes a dense desmoplastic reaction (23), which makes it difficult for immune cells to physically find the tumor and may also lead to low cellularity on biopsy specimens, which makes molecular subtyping and tumor analysis difficult. This may be related to the stimulation of KRAS as a response to pancreatic injury, which not only leads to development of neoplastic lesions, but also the desmoplastic environment (23). In addition, the TME is enriched in regulatory T cells and myeloid derived suppressor cells (MDSC) and lacking in cytotoxic T cells (CTL) that appropriately target tumor cells for destruction (23-29). Pancreatic tumors have also been known to secrete TGF-B, IL-10, indoleamine 2,3-dioxygenase, galectin-1, and express PD-L1, all of which function to impair an immune-response (30-33).
One possible immunotherapeutic approach, therefore, involves modifying the TME to facilitate immune responses. One method of doing this involves combining standard chemotherapeutic regimens with immune-stimulatory agents. This can be done either alone or in combination with standard of care chemotherapy. For example, the role for agonistic CD40 inhibition with gemcitabine and nab-paclitaxel is currently in question. The combination of gemcitabine/nab-paclitaxel has been found to be associated superior to single-agent gemcitabine in metastatic pancreatic cancer (34) and the use of CD40 antibody has been found to increase T-cell derived pancreatic cancer cell destruction and produce durable remissions in mouse models (35). Another example includes sildenafil, which works by suppressing regulatory T cell production while stimulating CTL production and appears to be a promising avenue for further research (36). Sildenafil also increases blood flow via phosphodiesterase-5 inhibition, thus potentially increasing tumor access. Another immune-stimulating approach focuses on modulating tumor-associated macrophage and MDSC responses. By blocking the colony stimulating factor 1/colony stimulating factor 1 receptor (CSF1/CSF1R) interaction on MDSCs, tumor antigen presentation may be enhanced and help produce anti-tumor T-cell responses (37). Similar interactions occur in the CCL2/CCR2 pathway, and this is the subject of a recently reported phase I clinical trial, where an oral CCR2 inhibitor (PF-04136309) was added to FOLFIRINOX in borderline or locally advanced pancreatic cancer (38). The combination was found to have acceptable toxicity and will move forward to further testing. This inhibition, however, also up-regulates T-cell checkpoint molecules such as PD-1, which may lead to apoptosis of cytotoxic T-cells (37). Therefore, it is suggested that CSF1/CSF1R inhibition may be best applied in conjunction with checkpoint molecule antagonists, such as PD-1/PD-1L inhibitors and will require further investigation as such (15,37). This approach was successful in a first-in human study of cabiralizumab and nivolumab, eliciting a response in 4 of 31 heavily pretreated patients (39). Further adding to the immune-suppressive environment is the increased expression of indoleamine 2,3-dioxygenase (IDO) in the TME. IDO is an enzyme that functions to catalyzing tryptophan, leading to T-cell arrest (40,41). Inhibition of this enzyme via indoximod, in combination with gemcitabine and nab-paclitaxel, was the subject of a recently reported phase I trial, and will be moving forward into phase II (42).
Anti-tumor vaccination
Anti-tumor vaccination is being investigated in order to generate the long-lasting, highly specific CTLs that are lacking in the PDAC TME. This can be accomplished with exogenously derived dendritic cells targeted to specific tumor cells (43). In patients with metastatic prostate cancer, sipuleucel-T (also known as APC8015) has been shown to improve patient outcomes and is currently approved for use in metastatic castrate-resistance prostate cancer (44). Other vaccines have also been shown to be effective in treating follicular lymphoma (45,46). Due to these successes, there have been several attempts at creating an anti-PDAC vaccine. One such attempt is the GVAX vaccine, a whole cell-based vaccine that consists of two pancreatic cell lines that have been engineered to express granulocyte macrophage colony-stimulating factor (GM-CSF) (23). This cytokine helps promote the growth and differentiation of dendritic cells, which works in step 2 (antigen presentation) of the cancer immunity cycle. In a randomized neoadjuvant/adjuvant clinical trial, the vaccine was shown to increase immunogenic T-cell infiltration into the TME, as well as increase expression of PD-1/PD-1L (47). Unfortunately, a phase IIb trial did not show improved survival with the use of GVAX, cyclophosphamide, and CS-207 (a live, attenuated form of listeria designed to express mesothelin, thus stimulating T cells both in the innate and adaptive immune system) over chemotherapy alone in metastatic patients (48). Algenpantucel-L is a whole-cell vaccine that involves allogeneic pancreatic cells that have been irradiated and made to express alpha-gal, an epitope normally not expressed in humans, and one against which the human gut continually produces anti-bodies (23). Exposure to alpha-gal, therefore, leads to hyperacute rejection (and potent immune-stimulation) via both complement and antibody-mediated immune responses (49-51). However, the IMPRESS trial has reportedly failed to show survival benefit in patients who received algenpantucel-L after resection (52). Though these vaccination strategies have shown promise in pre-clinical models, they have not yet demonstrated clinical benefit and may need to be further investigated as a part of a model that includes induction of novel innate immune reactions (radiation) and immune checkpoint inhibitors.
Gene-mediated cytotoxic immunotherapy (GMCI)
Finally, GMCI is being explored as another method for antigen presentation and oncolysis. One particular therapy being examined utilizes the herpes thymidine kinase gene, which will ideally specifically infiltrate tumor cells as a virus would and stimulate production of herpes signaling mechanisms. Valacyclovir can then be given in an attempt to kill infected tumor cells, thus targeting both the antigen presentation and cell killing portion of the immune response (Figure 1). Genetic sequences from other viruses and bacteria are also being investigated in small, phase I studies. Early results from some of these studies are promising (23). Finally, cyclophosphamide, a chemotherapeutic agent not normally used in PDAC, is also being investigated for its role in pancreatic cancer as it has been found to inhibit T regulatory cells (53)
The role of RT in pancreatic cancer and the immune response
The role of radiotherapy in pancreatic cancer, outside of its role in the cancer-immunity cycle, is controversial. In locally advanced pancreatic cancer, multiple trials have resulted over the last 30 years, with mixed results, and all have been criticized for various reasons. Indeed, the most recent trial LAP-07, found no benefit to the addition of radiotherapy to gemcitabine after four months of induction gemcitabine for locally advanced pancreatic cancer. In resectable pancreatic cancer, ESPAC-1 did not find a benefit to the addition of radiotherapy to adjuvant chemotherapy, though further studies are ongoing. Possible reasons for lack of efficacy in the definitive or adjuvant setting have included (I) underestimating the role for systemic therapy and (II) the difficulty with dose escalation given proximity to dose-limiting structures (stomach, liver, duodenum), leading to inadequate local treatment. Given the improvements in survival in metastatic pancreatic cancer with multi-agent regimens and improved survival seen in single-institutional datasets when multi-agent chemotherapy is followed by chemoradiotherapy, it follows that for local therapy to be effective, the systemic nature of the disease must be first addressed. Dose escalation and hypofractionation are increasingly being investigated as possible methods of improved local tumor control and, in combination with the increasing use of multi-agent chemotherapy, may increase the role for radiotherapy in pancreatic cancer. However, all randomized clinical trials that have currently resulted have utilized conventional radiotherapy [1.8–2 gray (Gy) per fraction over the course of several weeks] and no trial has been completed for dose escalation, leaving the role of dose escalation and altered fractionation unanswered.
The role of radiotherapy in the cancer-immunity cycle is increasingly being investigated, and may involve different specifications when the goal is immune-stimulation, as opposed to direct ablation of tumor cells while sparing critical organs. As such, the optimal regimen has not yet been established. Both in vitro and in vivo models have examined conventional fractionation (1.8–2 Gy per fraction), hypofractionation (>2 Gy per fraction for several fractions), and high-dose radiotherapy (>8 Gy per fraction, given as a single dose) in different cancer cell lines, with varying results. It is argued that in long courses of conventional, daily radiotherapy, increasing lymphocyte death occurs, limiting the immune response (54,55). Another group found that fractionated, as opposed to single, radiotherapy treatments were needed for induction of the abscopal response with anti-CTLA-4 drugs and that single, large doses of radiotherapy may be so ablative that they do not generate an immune response (56). Studies that involve combining radiotherapy with immunotherapy must be compared with not only the other agents used but also the method of delivery of radiotherapy, making comparison difficult.
Combination RT and immunotherapy—success in other cancer types
Though several of the previously described possible targets in inducing immune responses are quite promising, it is likely that no one option will be enough in itself, especially in tumors that are not as immunogenic as melanoma. Certainly, any amount of immune priming will not help if the immune system does not have a target—that is, if it has not been presented with an antigen. RT, which is thought to enhance antigen presentation, may play an important role in the immune response. By damaging the tumor cells, RT draws the attention of the body’s immune system, which may then recognize the tumor cells as foreign, triggering an adaptive response. Park et al. reported that when given with a PD-1 blocker, stereotactic body radiotherapy to melanoma and renal cell carcinoma tumors induced a 66% reduction in size of non-irradiated secondary tumors, as well as near complete regression of the primary tumor (57). Further case reports in melanoma patients suggest that adding radiotherapy to ipilimumab treatment may improve patient outcomes (58,59). Indeed, Grimaldi et al. show that use of RT following a standard dosage of ipilimumab led to improved survival compared to ipilimumab alone (22.4 vs. 8.3 months) (60). Similar effects have been noted in the combination of GM-CSF with radiotherapy to treat metastatic solid tumors (including small cell lung, non-small cell lung, breast, thymic, urothelial, ovarian, eccrine, and cervical cancers) (61). In this study, approximately 27% of patients experienced abscopal-like events, and in the patients who did, survival improved significantly (20.98 vs. 8.33 months) (61). Though melanoma is thought to be more immunogenic than pancreatic cancer, these fascinating results from both pre-clinical and clinical models has led to increased interest in combining radiotherapy with other immune-priming modalities in the treatment of all stages of pancreatic cancer.
Current clinical trials combining immunotherapy and RT in pancreatic cancer
The great majority of currently open trials in the stimulation of an adaptive immune response in pancreatic cancer seek to target multiple portions of the immune response, often with the combination of standard of care therapy (surgery, chemotherapy, and/or radiotherapy) with either immune checkpoint inhibitors or other immune-priming agents. These therapies are being investigated in all stages of pancreatic cancer, from early stage (resectable) disease to metastatic disease. These trials are also for the most part in the safety phase, and will still need to demonstrate efficacy, before they can begin to be investigated for a possible relationship to what is already considered standard-of-care.
The endpoints of these studies vary, from survival to disease control rate to tumor response in resected/biopsied tissue to predictive biomarkers in the blood. Biomarkers investigated as a part of the systemic immune response in general have included: TNF-alpha, IFN-beta, IL-12p70, IL-6, IL-8, as well as total circulating immune cells, and CD4+/CD8+ T-cells, to name a few (62). In the below trials, pre- and post-treatment CD8+ T cell and macrophage infiltration in tissues, peripheral blood T cells, CA-125, mesothelin-specific T cells, are also being investigated. They are listed where applicable.
Trials for resectable disease (63-76) (Table 1)
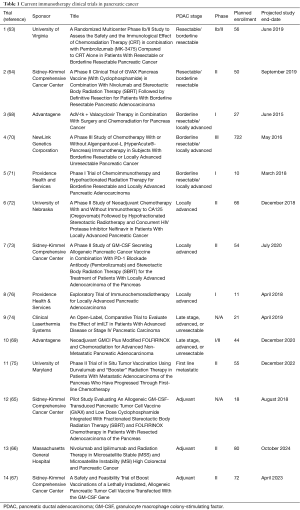
Full table
In resectable and borderline resectable pancreatic cancers, both neoadjuvant and adjuvant therapies are being evaluated, including the addition of pembrolizumab (MK-3475) to chemoradiotherapy in the neoadjuvant setting, with the endpoint being the number of tumor infiltrating lymphocytes per high powered field after resection and incidence of dose-limiting toxicities (DLTs) (Trial 1, Table 1) (63). Secondary endpoints will include disease free survival (DFS), OS, and RR. Another study will examine the addition of the GVAX vaccine, cyclophosphamide, nivolumab, and SBRT to resection in borderline resectable patients (Trial 2, Table 1) (64). The primary endpoint is pathologic complete RR (pCR), but other important endpoints include vaccine-induced changes in the number, function, avidity, size and diversity of the mesothelin-specific T-cells (64). The role for the GVAX vaccine and cyclophosphamide will also be examined in the adjuvant setting, with the first 6 patients receiving SBRT and FOLFIRINOX only after resection and the last 12 receiving SBRT and FOLFIRINOX in conjunction with cyclophosphamide and the GVAX vaccine (Trial 12, Table 1) (65). The primary endpoint will be toxicity and secondarily disease progression. Combined immune check-point inhibitors are also being assessed in the adjuvant setting: patients will receive both ipilimumab (CTLA-4 inhibitor) and nivolumab (PD-1 inhibitor) therapy along with radiotherapy with the primary endpoint being disease control rate, and secondarily DFS and OS (Trial 13, Table 1) (66). Finally, another adjuvant trial is being pursued aimed at examining the combination of a GM-CSF secreting vaccine targeted to pancreatic cancer cells with cyclophosphamide, with a primary endpoint of not only safety, but disease-free survival (Trial 14, Table 1) (67).
Trials for borderline resectable and locally advanced disease
Similar themes are emerging in currently open trials in borderline and locally advanced patients. One currently open phase I trial will involve GMIC given in addition to either standard of care surgery or chemoradiotherapy, depending on the patient’s resectability status (Trial 3, Table 1) (68). Pathology specimens will be examined for CD8+ tumor infiltration and CA 19-9 response. In another study, GMIC will be given specifically to locally advanced patients with mFOLFIRINOX (modified 5-fluorouracil, leucovorin, irinotecan, oxaliplatin), gemcitabine, radiation. Surgery will be considered if the patient becomes resectable by imaging (Trial 10, Table 1) (69). One of the questions examined in this trial is whether the addition of GMIC can aid in the conversion of previously unresectable patients to resectable. A currently open phase III trial studies the addition of algenpantucel-L to standard of care treatment for borderline and locally advanced PDAC (Trial 4, Table 1) (70). It will be added to one of two multi-agent chemotherapy regimens and compared to those chemotherapy regimens alone for OS and correlative lab studies will be collected, though not specified on the clinical trial website. Tadalafil, a phosphodiesterase-5 inhibitor like sildenafil, is actively being investigated in phase I status as an addition to chemoradiotherapy +/− surgery depending on resectability status (Trial 5, Table 1) (71). Primary endpoint includes not only safety, but also immune infiltration into surgical tissue and T-cell proliferation in the blood. Oregovomab, a CA-125 monoclonal antibody, is being studied in conjunction with an HIV protease inhibitor (nelfinavir) in patients who will be receiving chemotherapy and radiotherapy, with the goal of reporting the number of patients with disease progression, pathologic response, and serum CA-125 levels (Trial 6, Table 1) (72). In another study, 54 patients will receive the combination of cyclophosphamide, pembrolizumab, and the GVAX vaccine, as well as SBRT, thus potentially targeting several stages of the immune response (Trial 7, Table 1) (73). In addition to either conventionally fractionated radiotherapy or SBRT, immunostimulating interstitial laser thermotherapy (imILT) is also being investigated as a possible addition to standard chemotherapeutic treatment (Trial 9, Table 1) (74). In metastatic pancreatic cancer, researchers are evaluating whether the combination of conventionally fractionated, low-dose radiation will augment the anti-tumor immunogenic response elicited by durvalumab, as measured by PFS in metastatic patients (Trial 11, Table 1) (75). Similarly, an ongoing study is evaluating utilizing a telomerase vaccine in conjunction with a GM-CSF (sargramostim) injection, gemcitabine chemotherapy, RT, and tadalafil in order to determine the safety of this immune-chemoradiotherapy (Trial 8, Table 1) (76). Macrophage and T cell tumor infiltrates will be gathered, as well as CD8+ T cell counts pre- and post-therapy.
Summary and future directions
While survival has continued to improve in all stages of pancreatic cancer since the earliest clinical trials, it remains poor, even with aggressive treatment. There is an increasing body of research that suggests immunotherapy may be a promising option for a multitude of malignancies and researchers are just beginning to understand both tumor and patient-specific factors that may lead to the most effective treatment options. Though pancreatic cancer does not appear to respond as effectively to single-agent immune stimulation (whether by radiotherapy or immune-checkpoint inhibitors) as other more immunogenic cancers, this does not mean that the immune system cannot play a role in its treatment. Instead, several stages of therapies may be needed, including: countering the immunosuppressive microenvironment, increasing antigen presentation, and more effectively priming the immune system to respond. The great majority of currently open trials in the stimulation of an adaptive immune response in pancreatic cancer seek to target multiple portions of the immune response, often with the combination of standard of care therapy (surgery, chemotherapy, and/or radiotherapy) with either immune checkpoint inhibitors or other immune-priming agents. We look forward to the results of the clinical trials presented in this review, as well as the advancement of these therapies to phase II/III trials, in hopes that outcomes can be improved for this deadly disease.
Acknowledgements
None.
Footnote
Conflicts of Interest: S Lloyd has received funding from Sirtex Medical outside of the submitted work. The other authors have no conflicts of interest to declare.
References
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- The reference should be changed to National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology, Pancreatic Adenocarcinoma. 2017. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic_blocks.pdf
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Lowery MA, Jordan EJ, Basturk O, et al. Real-Time Genomic Profiling of Pancreatic Ductal Adenocarcinoma: Potential Actionability and Correlation with Clinical Phenotype. Clin Cancer Res 2017;23:6094-100. [Crossref] [PubMed]
- Finn OJ. Immuno-oncology: understanding the function and dysfunction of the immune system in cancer. Ann Oncol 2012;23 Suppl 8:viii6-9.
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged Survival in Stage III Melanoma with Ipilimumab Adjuvant Therapy. N Engl J Med 2016;375:1845-55. [Crossref] [PubMed]
- Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 2015;16:522-30. [Crossref] [PubMed]
- Weber J, Mandala M, Del Vecchio M, et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III or IV Melanoma. N Engl J Med 2017;377:1824-35. [Crossref] [PubMed]
- Reck M. Pembrolizumab as first-line therapy for metastatic non-small-cell lung cancer. Immunotherapy 2018;10:93-105. [Crossref] [PubMed]
- Reck M, Rodriguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Cohen EE, Harrington KJ, Le Tourneau C, et al. Pembrolizumab (pembro) vs standard of care (SOC) for recurrent or metastatic head and neck squamous cell carcinoma (R/M HNSCC): Phase 3 KEYNOTE-040 trial. Presented at ESMO 2017 Congress; September 8-12, 2017; Madrid, Spain.
- Cohen RB, Delord JP, Doi T, et al. Pembrolizumab for the Treatment of Advanced Salivary Gland Carcinoma: Findings of the Phase 1b KEYNOTE-028 Study. Am J Clin Oncol 2018. [Epub ahead of print]. [PubMed]
- Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 2015;23:32-8. [Crossref] [PubMed]
- Javle M, Golan T, Maitra A. Changing the course of pancreatic cancer--Focus on recent translational advances. Cancer Treat Rev 2016;44:17-25. [Crossref] [PubMed]
- Ebert PJR, Cheung J, Yang Y, et al. MAP Kinase Inhibition Promotes T Cell and Anti-tumor Activity in Combination with PD-L1 Checkpoint Blockade. Immunity 2016;44:609-21. [Crossref] [PubMed]
- Bendell JC, Bang YJ, Chee CE, et al. A phase Ib study of safety and clinical activity of atezolizumab (A) and cobimetinib (C) in patients (pts) with metastatic colorectal cancer (mCRC). J Clin Oncol 2018;suppl 4S:abstr 560.
- Kaminski JM, Shinohara E, Summers JB, et al. The controversial abscopal effect. Cancer Treat Rev 2005;31:159-72. [Crossref] [PubMed]
- Demaria S, Ng B, Devitt ML, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004;58:862-70. [Crossref] [PubMed]
- Reynders K, Illidge T, Siva S, et al. The abscopal effect of local radiotherapy: using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015;41:503-10. [Crossref] [PubMed]
- Hu ZI, McArthur HL, Ho AY. The Abscopal Effect of Radiation Therapy: What Is It and How Can We Use It in Breast Cancer? Curr Breast Cancer Rep 2017;9:45-51. [Crossref] [PubMed]
- Balachandran VP, Luksza M, Zhao JN, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017;551:512-6. [PubMed]
- McCormick KA, Coveler AL, Rossi GR, et al. Pancreatic cancer: Update on immunotherapies and algenpantucel-L. Hum Vaccin Immunother 2016;12:563-75. [Crossref] [PubMed]
- Hiraoka N, Onozato K, Kosuge T, et al. Prevalence of FOXP3+ regulatory T cells increases during the progression of pancreatic ductal adenocarcinoma and its premalignant lesions. Clin Cancer Res 2006;12:5423-34. [Crossref] [PubMed]
- Esposito I, Menicagli M, Funel N, et al. Inflammatory cells contribute to the generation of an angiogenic phenotype in pancreatic ductal adenocarcinoma. J Clin Pathol 2004;57:630-6. [Crossref] [PubMed]
- Dodson LF, Hawkins WG, Goedegebuure P. Potential targets for pancreatic cancer immunotherapeutics. Immunotherapy 2011;3:517-37. [Crossref] [PubMed]
- Sideras K, Braat H, Kwekkeboom J, et al. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev 2014;40:513-22. [Crossref] [PubMed]
- Ikemoto T, Yamaguchi T, Morine Y, et al. Clinical roles of increased populations of Foxp3+CD4+ T cells in peripheral blood from advanced pancreatic cancer patients. Pancreas 2006;33:386-90. [Crossref] [PubMed]
- Gabitass RF, Annels NE, Stocken DD, et al. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011;60:1419-30. [Crossref] [PubMed]
- Bellone G, Turletti A, Artusio E, et al. Tumor-associated transforming growth factor-beta and interleukin-10 contribute to a systemic Th2 immune phenotype in pancreatic carcinoma patients. Am J Pathol 1999;155:537-47. [Crossref] [PubMed]
- Fallarino F, Grohmann U, You S, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor zeta-chain and induce a regulatory phenotype in naive T cells. J Immunol 2006;176:6752-61. [Crossref] [PubMed]
- Geng L, Huang D, Liu J, et al. B7-H1 up-regulated expression in human pancreatic carcinoma tissue associates with tumor progression. J Cancer Res Clin Oncol 2008;134:1021-7. [Crossref] [PubMed]
- Nomi T, Sho M, Akahori T, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res 2007;13:2151-7. [Crossref] [PubMed]
- Von Hoff DD, Ervin T, Arena FP, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013;369:1691-703. [Crossref] [PubMed]
- Byrne KT, Vonderheide RH. CD40 Stimulation Obviates Innate Sensors and Drives T Cell Immunity in Cancer. Cell Rep 2016;15:2719-32. [Crossref] [PubMed]
- Bazhin AV, Bayry J, Umansky V, et al. Overcoming immunosuppression as a new immunotherapeutic approach against pancreatic cancer. Oncoimmunology 2013;2:e25736. [Crossref] [PubMed]
- Zhu Y, Knolhoff BL, Meyer MA, et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res 2014;74:5057-69. [Crossref] [PubMed]
- Nywening TM, Wang-Gillam A, Sanford DE, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol 2016;17:651-62. [Crossref] [PubMed]
- Wainberg ZA, Piha-Paul S, Luke JJ, et al. First-in-Human Phase 1 Dose Escalation and Expansion of a Novel Combination, Anti-CSF-1 Receptor (cabiralizumab) Plus Anti–PD-1 (nivolumab), in Patients With Advanced Solid Tumors. 32nd SITC Annual Meeting, 2017, National Harbor, MD.
- Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269-74. [Crossref] [PubMed]
- Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med 2004;10:15-8. [Crossref] [PubMed]
- Bahary N, Garrido-Laguna I, Wang-Gillam A, et al. Results of the phase Ib portion of a phase I/II trial of the indoleamine 2,3-dioxygenase pathway (IDO) inhibitor indoximod plus gemcitabine/nab-paclitaxel for the treatment of metastatic pancreatic cancer. J Clin Oncol 2016;34:452. [Crossref]
- Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265-77. [Crossref] [PubMed]
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010;363:411-22. [Crossref] [PubMed]
- Neelapu SS, Gause BL, Nikcevich DA, et al. Phase III randomized trial of patient-specific vaccination for previously untreated patients with follicular lymphoma in first complete remission: protocol summary and interim report. Clin Lymphoma 2005;6:61-4. [Crossref] [PubMed]
- Schuster SJ, Neelapu SS, Santos CF, et al. Idiotype vaccination as consolidation therapy: time for integration into standard of care for follicular lymphoma? J Clin Oncol 2011;29:4845-6. [Crossref] [PubMed]
- Lutz ER, Kinkead H, Jaffee EM, et al. Priming the pancreatic cancer tumor microenvironment for checkpoint-inhibitor immunotherapy. Oncoimmunology 2014;3:e962401. [Crossref] [PubMed]
- Le DT, Ko AH, Wainberg ZA, et al. Results from a phase 2b, randomized, multicenter study of GVAX pancreas and CRS-207 compared to chemotherapy in adults with previously-treated metastatic pancreatic adenocarcinoma (ECLIPSE Study). J Clin Oncol 2017;35:345. [Crossref]
- Hardacre JM, Mulcahy M, Small W, et al. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: a phase 2 study. J Gastrointest Surg 2013;17:94-100; discussion 101. [Crossref] [PubMed]
- Macher BA, Galili U. The Galalpha1,3Galbeta1,4GlcNAc-R (alpha-Gal) epitope: a carbohydrate of unique evolution and clinical relevance. Biochim Biophys Acta 2008;1780:75-88. [Crossref] [PubMed]
- Galili U, Anaraki F, Thall A, et al. One percent of human circulating B lymphocytes are capable of producing the natural anti-Gal antibody. Blood 1993;82:2485-93. [PubMed]
- Broderick JM. OncLive. Pancreatic Cancer Vaccine Falls Short in Phase III Trial. 2016. Accessed 3/16/18. Available online: http://www.onclive.com/web-exclusives/pancreatic-cancer-vaccine-falls-short-in-phase-iii-trial
- Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol 2004;34:336-44. [Crossref] [PubMed]
- Multhoff G, Gaipl US, Niedermann G. The role of radiotherapy in the induction of antitumor immune responses. Strahlenther Onkol 2012;188 Suppl 3:312-5. [Crossref] [PubMed]
- Frey B, Rubner Y, Wunderlich R, et al. Induction of abscopal anti-tumor immunity and immunogenic tumor cell death by ionizing irradiation - implications for cancer therapies. Curr Med Chem 2012;19:1751-64. [Crossref] [PubMed]
- Dewan MZ, Galloway AE, Kawashima N, et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009;15:5379-88. [Crossref] [PubMed]
- Park SS, Dong H, Liu X, et al. PD-1 Restrains Radiotherapy-Induced Abscopal Effect. Cancer Immunol Res 2015;3:610-9. [Crossref] [PubMed]
- Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012;366:925-31. [Crossref] [PubMed]
- Hiniker SM, Chen DS, Knox SJ. Abscopal effect in a patient with melanoma. N Engl J Med 2012;366:2035-author reply 2035-6. [Crossref] [PubMed]
- Grimaldi AM, Simeone E, Giannarelli D, et al. Abscopal effects of radiotherapy on advanced melanoma patients who progressed after ipilimumab immunotherapy. Oncoimmunology 2014;3:e28780. [Crossref] [PubMed]
- Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015;16:795-803. [Crossref] [PubMed]
- Deloch L, Derer A, Hartmann J, et al. Modern Radiotherapy Concepts and the Impact of Radiation on Immune Activation. Front Oncol 2016;6:141. [Crossref] [PubMed]
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02305186. A Randomized Multicenter Phase Ib/II Study to Assess the Safety and the Immunological Effect of Chemoradiation Therapy (CRT) in Combination With Pembrolizumab (MK-3475) Compared to CRT Alone in Patients With Resectable or Borderline Resectable Pancreatic Cancer. March 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02305186
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03161379. A Phase II Clinical Trial of GVAX Pancreas Vaccine (With Cyclophosphamide) in Combination With Nivolumab and Stereotactic Body Radiation Therapy (SBRT) Followed by Definitive Resection for Patients With Borderline Resectable Pancreatic Adenocarcinoma. May 19, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03161379
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03161379. Pilot Study Evaluating An Allogeneic GM-CSF-Transduced Pancreatic Tumor Cell Vaccine (GVAX) and Low Dose Cyclophosphamide Integrated With Fractionated Stereotactic Body Radiation Therapy (SBRT) and FOLFIRINOX Chemotherapy in Patients With Resected Adenocarcinoma of the Pancreas. May 10, 2012. Available online: https://clinicaltrials.gov/ct2/show/NCT01595321
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03104439. Nivolumab and Ipilimumab and Radiation Therapy in Microsatellite Stable (MSS) and Microsatellite Instability (MSI) High Colorectal and Pancreatic Cancer. April 7, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03104439
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01088789. A Safety and Feasibility Trial of Boost Vaccinations of a Lethally Irradiated, Allogeneic Pancreatic Tumor Cell Vaccine Transfected With the GM-CSF Gene. March 17, 2010. Available online: https://clinicaltrials.gov/ct2/show/NCT01088789
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT00638612. AdV-tk + Valacyclovir Therapy in Combination With Surgery and Chemoradiation for Pancreas Cancer. March 19, 2008. Available online: https://clinicaltrials.gov/ct2/show/NCT00638612
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02446093. Neoadjuvant GMCI Plus Modified FOLFIRINOX and Chemoradiation for Advanced Non-Metastatic Pancreatic Adenocarcinoma. May 18, 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02446093
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01836432. A Phase III Study of Chemotherapy With or Without Algenpantucel-L (HyperAcute®-Pancreas) Immunotherapy in Subjects With Borderline Resectable or Locally Advanced Unresectable Pancreatic Cancer. April 19, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01836432
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01903083. Phase I Trial of Chemoimmunotherapy and Hypofractionated Radiation Therapy for Borderline Resectable and Locally Advanced Pancreatic Adenocarcinoma. June 19, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01903083
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01959672. A Phase II Study of Neoadjuvant Chemotherapy With and Without Immunotherapy to CA125 (Oregovomab) Followed by Hypofractionated Stereotactic Radiotherapy and Concurrent HIV Protease Inhibitor Nelfinavir in Patients With Locally Advanced Pancreatic Cancer. October 10, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT01959672
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02648282. A Phase II Study of GM-CSF Secreting Allogeneic Pancreatic Cancer Vaccine in Combination With PD-1 Blockade Antibody (Pembrolizumab) and Stereotactic Body Radiation Therapy (SBRT) for the Treatment of Patients With Locally Advanced Adenocarcinoma of the Pancreas. January 7, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02648282
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT03187587. An Open-Label, Comparative Trial to Evaluate the Effect of imILT in Patients With Advanced Disease or Stage IV Pancreatic Carcinoma. June 15, 2017. Available online: https://clinicaltrials.gov/ct2/show/NCT03187587
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT02885727. Phase II Trial of in Situ Tumor Vaccination Using Durvalumab and "Booster" Radiation Therapy in Patients With Metastatic Adenocarcinoma of the Pancreas Who Have Progressed Through First-line Chemotherapy. August 31, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02885727
- ClincialTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29. Identifier NCT01342224. Exploratory Trial of Immunochemoradiotherapy for Locally Advanced Pancreatic Adenocarcinoma. April 11, 2011. Available online: https://clinicaltrials.gov/ct2/show/NCT01342224