The role of radiotherapy in management of pancreatic cancer
Department of Radiation Oncology, University of Kansas Medical Center, Kansas City, Kansas, USA
|
Review Article
The role of radiotherapy in management of pancreatic cancer
Department of Radiation Oncology, University of Kansas Medical Center, Kansas City, Kansas, USA
|
|
Abstract
Pancreatic cancer is one of the leading causes of cancer death. The treatment options in pancreatic cancer remain limited.
This review provides an overview of the role of radiotherapy (RT) alone or in combination with systemic treatment at different
settings of treatment strategy. Neoadjuvant chemoradiotherapy (CRT) may downstage the borderline resectable
disease and make resection possible, which could translate to a survival benefit. Although the benefit of adjuvant CRT
remains controversial due to inconsistent outcome of randomized trials, in North America it is still a common recommendation
of the treatment. For locally advanced pancreatic cancer, the treatment option could either be chemotherapy
or chemoradiotherapy. By using advanced radiotherapy modalities, the toxicity of RT could be reduced and RT dose escalation
becomes possible to improve locoregional control.
Key words
pancreatic cancer, chemoradiotherapy, radiotherapy
J Gastrointest Oncol 2011; 2: 157-167. DOI: 10.3978/j.issn.2078-6891.2011.032
|
|
Introduction
Pancreatic cancer is the 10th most commonly diagnosed
cancer and the 4th leading cause of cancer death in the U.S.
An estimated 43,140 new cases were diagnosed and 36,800
deaths occurred in the U.S. in 2010. The survival rate for
this deadly disease has not improved substantially in nearly
the last 40 years even with aggressive treatment. For all
stages combined, the 1 and 5-year relative survival rates
are 25% and 6%, respectively. For patients diagnosed with
local disease, the 5-year survival is only 22% (1). Improving
outcomes for patients diagnosed with pancreatic cancer
continues to be a formidable challenge.
Surgical resection (pancreaticoduodenectomy) currently
provides the best opportunity for long-term survival.
However, only 10-20% of patients have resectable disease
at the time of diagnosis. The prognosis of patients after
complete resection is still poor, with a 3-year diseasespecific
survival rate of only 27% and a median survival
of only15-19 months (2-4). Locally advanced pancreatic
cancer (LAPC), in which the tumor encases the celiac axis or superior mesenteric artery with or without nodal disease
but without distant metastases, is by definition unresectable
and represents about 25% of the cases at diagnosis. For
these patients with LAPC, treatment usually consists of
chemotherapy (CT) alone or chemotherapy combined
with radiation (CRT), with a resultant median survival
only 10-12 months (5-7). Moreover, patients with limited
vascular involvement by tumor are considered to have
borderline resectable disease and are often treated with nonsurgical
therapy such as CT alone or CRT.
Patterns of failure data in pancreatic cancer treated with
surgical resection alone show that locoregional recurrence
is a large component of failure in 50% to 75% of cases
(8,9). In addition, hepatic and distant metastases rate is
approximately up to 85% to 90% coincident with evidence
of locoregional failure. Even in the series that patients
received adjuvant treatment after surgery, the locoregional
recurrence rate is still as high as 30% - 60% (10,11). Hence,
these patterns of failure indicate that current local and
systemic treatments are inadequate and there is significant
room for improvement.
Traditionally, radiation therapy as local treatment
has been utilized as neoadjuvant, adjuvant or definitive
treatment with or without systemic therapy. Anywhere
from approximately 20% to 80 % of the patients received
radiation therapy during the course of their treatment
(12). In several other disease sites “models” with high risk
of both locoregional and systemic failure, the additional
local radiotherapy to systemic chemotherapy has
demonstrated improvement of local control and overall survival. Representative examples include gastric cancer
and limited stage small cell lung cancer, among others,
in which the additional of local radiotherapy reduced the
risk of local-regional failure which eventually lead to a
decrease in systemic relapses and an improvement in overall
survival (13-18). Because of the patterns of recurrence in
pancreatic cancer include both locoregional failure in the
abdomen and systemic metastasis including the liver; it is
logical to consider both local radiotherapy and systemic
chemotherapy in the treatment of this cancer. The addition
of adjuvant chemoradiation has been reported to decrease
local recurrence rates to 20% – 40% (19,20) with some
studies even reporting local recurrence rates as low as 10%
(21–24). To prospectively evaluate the role of radiotherapy
on pancreatic cancer treatment, several randomized trials
have been conducted with conflicting results. Hence,
the routine utilization of radiation for pancreatic cancer
remains controversial.
This review will discuss the role of rationale for using
radiation therapy (RT) in the management of pancreatic
cancer, review the relevant literature, and discuss current
ongoing research and future directions.
|
|
Neoadjuvant radiotherapy
A neoadjuvant treatment strategy in pancreatic cancer may
offer several theoretical advantages: 1. Pancreatic cancer
is more likely a systemic disease with high incidence of
distal and local regional failure (10,11). By starting systemic treatment early we may be able to reduce the incidence of
distal metastasis and improve survival. 2. Neoadjuvant
radiotherapy with or without systemic therapy may
potentially downstage the disease and increase likelihood
of a complete resection (R0 resection). 3. Radiotherapy
can be better tolerated because the normal anatomy of the
abdominal region by surgery, such as bowel displacement,
which could lead to higher gastrointestinal toxicity, has
not been distorted. 4. Neoadjuvant radiotherapy can avoid
treating hypoxic tumor tissue caused by surgical disruption
of blood supply to tumor cells. In addition, cytokine
stimulation after surgery can also potentially adversely
affects the efficacy of adjuvant treatment, which can be
avoided by neoadjuvant RT (25). 5. Neoadjuvant treatment
may also identify those patients with aggressive disease who
are likely to develop early metastatic disease, and therefore
avoid unnecessary definitive surgical therapy. Given these
various rationales for neoadjuvant treatment, several
institutions have used this strategy in an effort to improve
the survival outcome of patients with pancreatic cancer
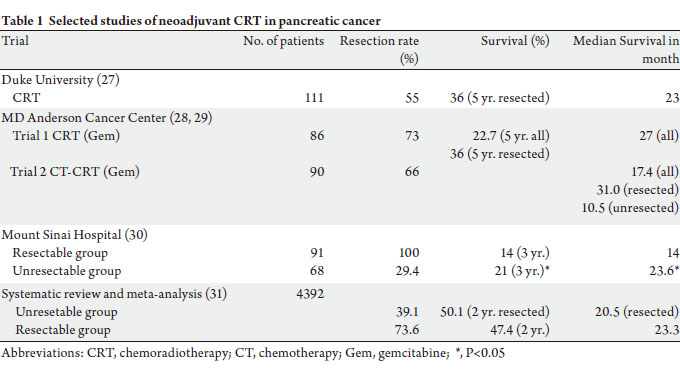
(Table 1). However, there have been no large randomized
controlled trials on the use of neoadjuvant therapy in
resectable pancreatic cancer.
The Duke University study investigated neoadjuvant
CRT in 96 resectable patients. Patients received dailyfractionated
radiotherapy to a total dose of 50.4 Gy
concurrent with 5-FU-based chemotherapy. Patients were
then re-staged after completion of CRT. Patients were then
surgically explored if there was no evidence of metastatic disease. Subsequently, 70% of patients underwent surgery
and 55% had a resection. A R0 resection was achieved in
75% of patients and operative mortality was 3.8%. Overall
survival (OS) for resected patients was 28% at 5 years, and a
median survival was 23months (26,27).
MD Anderson Cancer Center reported their neoadjuvant
treatment results using two different treatment strategies. In
their first trial, patients received neoadjuvant gemcitabine
and radiotherapy followed by surgery. Radiotherapy was
given concurrently with 7 doses of weekly gemcitabine to a
total dose of 30 Gy in 10 fractions. Of the 86 patients treated
from 2004 to 2006, 64 (73%) underwent resection with an
89% R0 resection rate. The perioperative complication was
9%. The median survival and 5 years OS for all 86 patients
were 22.7months and 27%, respectively. Patients, who
underwent a resection, did better with a 5 year OS of 36%
(28). The second trial was built up on this initial treatment
regimen using neoadjuvant combination of chemotherapy
prior to of CRT in an attempt to reduce distant metastasis
and improve OS (29). Ninety patients were enrolled into
this trial. Two cycles of cisplatin and gemcitabine were
given before concurrent CRT. Gemcitabine was used
for concurrent CRT. Sixty-two patients were deemed
radiologically resectable and underwent exploratory surgery.
A resection was completed in 52 (66%) patients. Positive
margins were found in 1 patient (R1 resection rate of 4%)
and nodal disease found in 58% of patients undergoing
successful resection. Median follow-up was 29.3 months.
The median survival was 17.4 months for all patients and
31 months for those undergoing resection. 27 patients who
did not undergo surgical resection had a median survival of
10.5 months. The investigators concluded that the addition
of induction cisplatin and gemcitabine chemotherapy prior
to neoadjuvant CRT did not improve OS.
In a prospective clinical trial comparing neoadjuvant
therapy to up-front surgery conducted at Mount Sinai
Hospital in New York City (30), laparotomy and/or CT
followed by EUS, angiography or laparoscopy was used
to determine potential respectability prior to therapeutic
intervention. Sixty-eight patients with locally invasive
non-resectable tumors were treated with split-coursechemoradiotherapy
(5-FU, streptozotocin and cisplatin)
and subsequent surgery if rendered amenable to resection.
Thirty of them underwent surgery with downstaging
observed in 20 patients. Ninety-one patients with resectable
tumors underwent immediate pancreaticoduodenectomy.
Sixty-three of them received adjuvant radiotherapy or
chemotherapy. The median survival and 3-year OS of all
patients receiving preoperative treatment were 23.6 months
and 21% compared to 14.0 months and 14% for patients who
had initial tumor resection (p = 0.006), respectively.
Recently, a systematic review and meta-analysis of
neoadjuvant therapy in 4,394 patients showed that those
patients with initial unresectable tumor but who underwent
resection after neoadjuvant treatment had comparable
survival (median overall survival 20.5 months) to patients
with initially resectable tumors (median overall survival
23.3 months) (31). This met-analysis included 111 trials
with total of 4,394 patients. Neoadjuvant chemotherapy
was given in 96.4% of the studies with the main agents
consisting of gemcitabine, 5-FU (and oral analogues),
mitomycin C, and platinum compounds. Neoadjuvant
radiotherapy was used in 93.7% of the studies with doses
ranging from 24 to 63 Gy. Approximately one third of the
initial unresectable tumors were resected after neoadjuvant
therapy. For patients with resectable tumors, resection and
survival rates after neoadjuvant therapy are similar to the
ones observed in “up-front” resected tumors that are treated
by adjuvant therapy.
Thus, in spite of decades of investigation of neoadjuvant
therapy in pancreatic cancer, there is currently no evidence
to support its routine use in clinical practice. However, the
available data suggest that patients with locally advanced
and/or unresectable tumors should be included in
neoadjuvant clinical trials and subsequently be evaluated
for resection (31).
 |
|
Adjuvant radiotherapy
The high incidence of locoregional and systemic failure
after resection in pancreatic cancer indicates the need
for effective adjuvant treatment (8). The role of adjuvant
radiotherapy is controversial due to the conflicting results
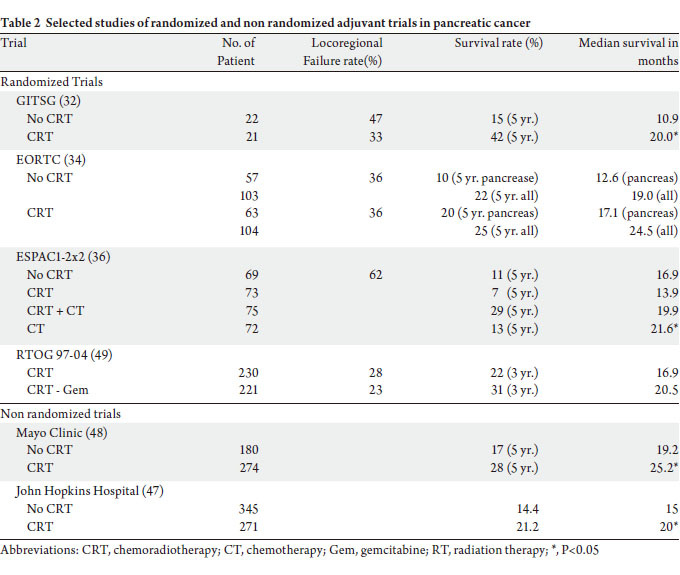
from the randomized controlled trials (Table 2).
The Gastro-intestinal Tumor Study Group (GITSG)
conducted first randomized trial in 1980’s to evaluate the
role of adjuvant CRT in resected pancreatic cancer. Fortynine
patients after R0 resection were randomized to CRT
versus observation (32). Radiotherapy was delivered to 40
Gy in 20 fractions with a planned 2-week break after 20 Gy.
Bolus fluorouracil (5-FU) was given concurrently and two
more cycles after radiotherapy. The treatment arm yielded
significantly longer median OS (20 vs. 11 months) and
2-year OS (42% vs. 15%) than the observation arm. Due to
this significant improvement in survival, thirty additional
patients were treated by the GITSG in a nonrandomized
fashion using an identical CRT regimen. The outcome
was similar to the treatment arm in the randomized trial
(33). Thus, the adjuvant CRT became a standard treatment
option for patients with resected pancreatic cancer in North
America.
In contrast, the adjuvant chemotherapy is considered the standard care for patients with resected pancreatic
cancer in Europe because the subsequent randomized
trials did not confirm the benefit of adjuvant CRT upon
survival (34,36,41). In the European Organization of
Research and Treatment of Cancer (EORTC) study, 218
patients with pancreatic or periampullary cancer were
randomized to CRT versus observation after resection
(34). The RT was delivered in the same fashion as in the
GITSG trial. Infusion 5-FU was substituted for bolus 5-FU
and no maintenance chemotherapy was administered. The
median survival in the subset of patients with pancreatic
cancer was 17.1 months in the CRT arm versus 12.6
months in the observation arm, a difference that did not
reach statistical significance (P = 0.099). An update of this
trial with longer median follow up of 11.7 years further
confirmed the absence of a statistical significant advantage
for adjuvant CRT (35). The ESPAC-1 (European Study
Group for Pancreatic Cancer) was a randomized trial in a 2 x 2 factorial design. After surgical resection, 289 patients
were assigned to observation, CT alone, CRT, or CRT
followed by CT (36). In addition, investigators had the
option of enrolling patients in 2 similar concurrent trials
(one testing CRT vs. observation and one testing CT
alone vs. observation), and the data across the 3 trials were
pooled for analysis. CRT regimen was similar to those of
the GITSG and EORTC trials although the total radiation
dose could be 40 or 60 Gy at the discretion of the treating
physician. The results showed a beneficial effect of adjuvant
CT upon OS, but a deleterious effect of CRT on survival. A
more recent analysis included only patients from the 2 x 2
factorial design trial and again showed a benefit for adjuvant
chemotherapy (37).
The results of three historical trials evaluating
concurrent chemo-radiotherapy (CRT) are confounded
by poor design of the trials, sub-optimal compliance of
the intended therapy and analysis. The GITSG study was criticized for slow accrual, small sample size, and
suboptimal radiotherapy with a low dose delivered in a splitcourse
fashion. The EORTC trial also employed suboptimal
radiotherapy similar to the GITSG study. The omission of
maintenance 5-FU, small sample size, high proportion of
patients forgoing the assigned therapy, and the inclusion of
patients with positive surgical margins without stratification
were all considered as study design flaws (38). In addition, it
has been argued that statistical significance of this possible
benefit is achieved with a one-sided log-rank test, which
could have been justified at the time this trial was designed
(P = 0.049) (39). The ESPAC-1 trial has been strongly
critiqued for allowing uncontrolled and previous therapy
in a substantial number of patients, introducing a selection
bias in the enrollment process and using suboptimal
radiotherapy (40). There was also a high rate of noncompliance
to the treatment regiments, which questions the
validity of any analysis and therefore its conclusions (42).
As mentioned above, all trials employed an outdated
radiotherapy regimen using low doses and a split-course
delivery; and there was absence of central radiation quality
control. All of these factors could have easily adversely
impacted the outcomes against the CRT arms. As evidence
for this adverse impact, a recent secondary analysis of
the Radiation Therapy Oncology Group (RTOG) 97-04
clinical trial showed that failure to adhere to prospectively
designated criteria for radiotherapy delivery was associated
with inferior survival (43).
The above available randomized trials have generated
conflicting results, and so the role of adjuvant CRT remains
controversial. In light of this dilemma, several recent
studies analyzed survival outcomes in patients who did or
did not receive postoperative RT using the Surveillance,
Epidemiology, and End Results (SEER) database (44-46).
Although each of these studies suffers from possible pitfalls
inherent in any retrospective analysis, these analyses have
the advantage of long follow up and large patient numbers,
which permit subgroup analyses not previously possible
with the randomized trials (46). Hazard and colleagues
[44] examined the effect of RT in resected pancreatic cancer
patients. On multivariate Cox regression analysis, a survival
benefit was noted in patients with T3, N1 disease. No
survival benefit, however, was seen for tumors limited to the
pancreas. A subsequent study by Artinyan and colleagues
(45) examined the role of adjuvant RT in a smaller patient
population with only node-negative disease. The survival
benefit associated with adjuvant RT was observed with
hazard ration (HR) of 0.87(95% CI, 0.75–1.00). The latest
SEER study by Moody and colleagues (46) included 3252
patients who underwent resection of nonmetastatic disease;
the adjuvant RT was associated with increase survival (HR, 0.87; 95% CI, 0.80–0.96). On subgroup analysis, only stage
IIB (T1-3N1) patients had a statistically significant benefit
associated with RT (HR, 0.70; 95% CI, 0.62–0.79). The
age of the patient and stage of disease were identified as
independent factors associated with RT use, which means
the younger patients with more advanced disease were more
likely to receive RT.
Fur thermore, two large nonrandomized studies
also suggested a survival benefit with adjuvant CRT in
pancreatic cancer (Table 2). A prospective study from Johns
Hopkins Hospital analyzed 616 pancreatic cancer patients,
who underwent surgery. Adjuvant CRT was associated with
improved median, 2- and 5-year survivals compared with
no CRT (47). Similarly, the Mayo Clinic reported their
3-decade experience of adjuvant therapy in 466 patients,
who underwent R0 resection. Adjuvant CRT significantly
improved median, 2- and 5-year survival compared with
surgery alone. Patients who received CRT had more adverse
prognostic factors than that not receiving adjuvant therapy
(48). The radiotherapy dose was 50.4Gy in both studies.
Unlike previous discussed trials, the Radiation Therapy
Oncology Group (RTOG) 97-04 (49) evaluated the
efficacy of gemcitabine in the adjuvant setting compared
to 5-Fluorouracil (5-FU). 451 patients were randomized
to pre- and post-CRT 5-FU versus pre- and post-CRT
gemcitabine after resection of pancreatic cancer. Univariate
analysis showed no difference in OS. Pancreatic head tumor
patients (n = 388) had a median survival and 5-year OS of
20.5 months and 22% with gemcitabine versus 17.1 months
and 18% with 5-FU, respectively. On multivariate analysis,
patients on the gemcitabine arm with pancreatic head
tumors experienced a trend toward improved OS (P = 0.08).
The local recurrence was 28% and the distant relapse rate
was 73%. Despite local recurrence being approximately half
of that reported in previous adjuvant trials, distant disease
relapse still occurred in ≥ 70% of patients. To address the
issue of high rate of distant metastasis and further define
the role of radiotherapy in adjuvant setting, the current
EORTC/U.S. Intergroup RTOG 0848 phase III adjuvant
trial evaluates the impact of targeted therapy Erlotinib and
CRT on OS after completion of a full course of gemcitabine.
The impact of adjuvant CRT vs. CT on outcome of
pancreatic cancer is another end point of this study.
 |
|
Definitive radiotherapy in locally advanced
pancreatic cancer
Thirty percent of patients present as locally advanced
pancreatic cancer (LAPC) at time of diagnosis (1).
The definition of LAPC is unresectable disease in the
absence of distant metastases. But in practice, borderline respectable tumor should be regarded as LAPC because of
the high likelihood of achieving an incomplete (R1 or R2)
resection. Patients with LAPC are potentially curable if a
R0 resection (R0) can be performed after downstaging of
the tumor, therefore it should be treated with the intention
of delivering curative therapy (31). Quite often, LAPC
is treated with chemotherapy, which improves quality
of life and survival when compared with best supportive
care (50). The additional local treatment with RT may
slow the progression of local disease and offer palliation
and /or prevention of of symptoms, such as pain, biliary
obstruction, bleeding, or bowel obstruction. When
chemotherapy is combined with RT, long-term survival has
been reported (51). However, the role of radiotherapy in
LAPC still remains undefined.
The advantage of CRT over best supportive care was
studied in a small prospectively randomized trial (52). 16
patients received CRT and 15 had supportive care. The
RT dose was 50.4 Gy (ranged from 25.2 to 60 Gy) and CT
was continues infusion 5-FU at 200 mg/m2/d. The median
survival was 13.2 months for CRT group vs. 6.4 months
for support care. The study demonstrated significant
improvement of OS and quality of life in the patients
received CRT.
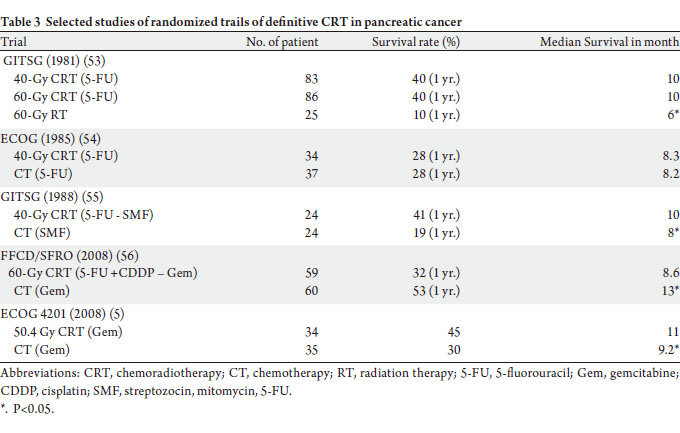
Early GITSG randomized trial compared combined
CRT (using RT doses of 40 Gy and 60 Gy with 5-FU) followed by additional CT vs 60 Gy RT alone (53).
Combined CRT was significantly superior to radiotherapy
alone, with mean OS times of 10.4 vs. 6.3 months. Higher
dose (60 Gy) of radiotherapy did not improve OS compared
to 40 Gy, although this may have been also a function of the
old delivery technique (2-D) of RT. This study established
general consensus that radiotherapy should be given
concurrently with chemotherapy in patients with LAPC.
Several subsequent randomized trials have compared
chemotherapy alone to CRT in LAPC, including 2 ECOG
trials (1989, 2008), 1 GITSG trial (1988), and 1 trial by the
Fondation Francophone de Cancerologie Digestive and
Societe Francaise de Radiotherapie Oncologique (FFCD/
SFRO) (Table 3) (54,5,55,56). Two studies (ECOG 1985
and FFCD/SFRO) showed no survival benefit to CRT. It
should be noted that radiotherapy delivery in ECOG 1985
trial was sub-optimal with split-course RT technique; and
FFCD/SFRO trial used unusually high dose radiotherapy
and non-standard chemotherapy regimen (5-FU and
cisplatin) in this setting with increasing toxicity. The
GITSG (1988) study and the ECOG 4021 demonstrated
survival benefit to CRT. The split-course of radiotherapy
and more toxic chemotherapy regimen (streptozotocin,
mitomycin, and 5-FU) used in GITSG (1980) could have
adversely affected the study outcome. The ECOG4201 is
only study using modern radiotherapy techniques (3-D conformal radiotherapy) and more effective chemotherapy
gemcitabine (5). Thirty-eight patients were treated with
gemcitabine alone and 36 with gemcitabine-based CRT. The
dose of radiation was 50.4 Gy. The results showed a small
but significant 2-month improvement in median survival
with the addition of RT (11.0 months vs. 9.2 months,
P<0.05). The median time to progression was also improved
with RT. Although the trial accrued only 74 out of 316
patients as study planned, the results suggest that there may
be a role for RT in patients with locally advanced disease, in
conjunction with gemcitabine chemotherapy.
 |
|
Advances in radiotherapy
In majority of the trials published before the early
1990s, conventional RT with larger fields of radiation
encompassing the pancreas or pancreatic bed and regional
nodes with margin were used. The use of this large volume of
radiation fields contributed to high incidence of GI toxicity,
especially when concurrent chemotherapy was employed.
Three-dimensional conformal radiotherapy (3-DRT), which
uses acquired CT images to allow delineation of target
volumes and precise localization of normal structures,
provides optimum coverage of the target and maximal
sparing of surrounding normal critical organs and tissues.
Intensity modulation radiation therapy (IMRT) is a more
recent advance in the delivery of RT. It generates more
conformal coverage of RT on target and maximizes the
sparing normal tissue than 3-DRT. University of Maryland
treated 46 patients with adjuvant CRT using IMRT (57).
The RT field included elective nodal areas. All patients
received CRT based on 5-FU in a schema similar to RTOG
97-04. Rates of acute gastrointestinal (GI) toxicity from
this study were compared with those from RTOG 97-04,
where all patients were treated with 3-DRT (Figure 1A and B). The overall incidence of Grade 3–4 acute GI toxicity was
significant lower in patients receiving IMRT-based CRT
compared with patients who had 3-DRT. With IMRT, it
is possible to deliver doses of 45 to 50 Gy to the typically
larger RT fields while escalating the dose to the tumor bed
to 54 to 60 Gy (58). Such dose escalation may be necessary
for patients with high risk of local recurrence. The higher
dose of radiation integrated with newer chemotherapeutic
and targeted agents, may be needed to improve both local
control as well as overall outcome in this subset of patients.
Several other methods for precise targeting and dose
escalation have been studied, including stereotactic body
radiation therapy (SBRT). SBRT delivers 1 to 5 ablative
doses of radiation to small area only including gross disease
with tight margin, as opposed to conventional fractionation
of 25 to 28 lower-dose fractions to a large field over normal
tissue to cover microscopic extension of disease and regional
lymph nodes. The studies using SBRT have demonstrated
high rate of feasibility with high rate of local control, but
with increase toxicity (Figure 1C) (59-62). In a phase II
study, SBRT was give to total dose of 30 Gy in 3 fractions
to unresectable pancreatic carcinoma [62]. The local
control rate was 57%; however, small-bowel toxicity was
high (18%), consisting of severe GI mucositis/ ulceration,
alone with a 4.5% perforation rate. In a trial conducted at
Stanford University, single dose of 25 Gy SBRT was given
to a small radiation field. An 84% local control rate at 12
months was reported with 4% grade 2 late toxicity and 9%
grade 3 or 4 late GI toxicity (60). Mahadevan et al. reported
their experience on SBRT using 3 fractions to total dose of
24 -36 Gy (61). After SBRT, patients received gemcitabine
for 6 months or until tolerance or disease progression. On
36 patients with median follow up 24 months, the local
control rate was 78% and the median survival was 14.3
months. Seventy-eight percent of patients developed distant metastasis. There were 25% grade II and 14% grade III
GI toxicity. The other application of SBRT in LAPC is to
boost primary tumor site after conventional radiotherapy
with or without chemotherapy. The Stanford University
group (62) enrolled 19 patients onto a prospective study to
evaluate this boost concept. 25 Gy single fraction SBRT was
delivered to primary tumor site after 45Gy of conventional
radiotherapy delivered in 5 weeks. The local control rate was
94% with 12.5% incidence of late duodenal ulcers. Although
the local control rate have been impressive, given the higher
rates of GI toxicities and that improved local control has
not translated into a survival benefit in these trials, caution
should be exercised in using this type of approach.
RT field size is a current topic of interest and research,
especially given the increasing interest in dose escalation
and more intensity of systemic treatment. Historically,
radiation fields have been large, encompassing the pancreas
or pancreatic bed with a 2- to 3-cm margin and including
lymph node regions, which may be harboring microscopic
disease. Growing evidence from other tumor models such
as non-small cells lung cancer suggests that small-involved
field radiation may be reasonable without compromising
local regional control and overall survival (63,64). In a
phase I trial of full-dose concurrent gemcitabine and smallinvolved
field radiotherapy for LAPC, there was only 1 of
23 patients developed regional nodal recurrence. This trial
showed that smaller RT field size might be reasonable (63).
In another study using involved field radiation concurrently
with full dose of capecitabine 500-600 mg/m2 twice daily,
the local and locoregional progression were 14% and10%,
respectively. 14 % patients presented with local and systemic
disease. There was only one patient who had grade III GI
toxicity (64). Although these data are encouraging, the
further investigation is still necessary to confirm the use of
involved small field of radiation.
|
|
Conclusion
The treatment of pancreatic cancer remains challenging.
The dismal outcome after various therapeutic strategies
highlights the need for continued study of optimizing
current treatment and incorporating novel agents
into existing regimens. The use of chemotherapy and
particularly radiotherapy are controversial because of
difficulties interpreting the available randomized data. In
neoadjuvant setting, there is no evidence to support routine
use of neoadjuvant CRT for resectable disease. However,
some patients with borderline resectable pancreatic cancer
may benefit from neoadjuvant CRT if the resection can be
performed. The assessment of resectability after neoadjuvant
CRT is critical to determining the need for surgery, which can have a significant impact on patient survival. With
advanced diagnostic images such as CT scan, MRI, PET
scan EUS, even minimal invasive procedure of laparoscopy,
it is possible to select out such patients, who can be benefit
from R0 resection. Newer techniques of delivering RT such
as IMRT and SBRT offer the opportunity to improve the
efficacy of neoadjuvant treatment due to its better tolerance
with chemotherapy and the potential for RT dose escalation.
In the adjuvant setting, CRT is still considered as a standard
treatment option in North America. But if an R0 resection
can be achieved, only chemotherapy can be recommended.
Currently, a reasonable therapeutic strategy in the adjuvant
and the definitive settings includes an initial 2 to 4 months
of gemcitabine-based chemotherapy, followed by restaging
and delivery of 5-FU–based CRT, or gemcitabine-based
CRT using 3-DRT or IMRT to involved fields. Further
investigations are needed to define more clearly the optimal
timing of radiotherapy, dose, field size, and technique. In
addition, the employment of more potent systemic agents,
including those with radiosensitizing properties may further
enhance the efficacy of RT (65). Several phase I/II trials
are exploring the efficacy of targeted agents and alternative
chemotherapeutic agents (66). ACOSOG Z05031, a phase
II trial using cisplatin, 5-FU and α-interferon, has shown
promising 2-year OS rate of 55% of and a median survival
of 27.1 months (67). Currently, on going RTOG 0848 phase
III adjuvant trial is evaluating impact of Erlotinib with CRT
on survival in pancreatic cancer.
|
|
References
Cite this article as:
Wang F, Kumar P. The role of radiotherapy in management of pancreatic cancer. J Gastrointest Oncol. 2011;2(3):157-167. DOI:10.3978/j.issn.2078-6891.2011.032
|