Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience
1Department of Gastroenterology & Hepatology; 2Department of Surgery, Indiana University, Indianapolis, Indiana, USA
|
Original Article
Fluid analysis prior to surgical resection of suspected mucinous pancreatic cysts. A single centre experience
1Department of Gastroenterology & Hepatology; 2Department of Surgery, Indiana University, Indianapolis, Indiana, USA
|
|
Abstract
Objective: EUS-FNA cytology and fluid analysis are frequently utilized to evaluate pancreatic cysts. Elevated cyst fluid CEA is usually indicative of a mucinous pancreatic cyst but whether CEA or amylase values can subclassify various mucinous cysts is unknown. The purpose of this study is to determine whether cyst fluid CEA and amylase obtained by EUS-FNA can diff erentiate between mucinous cystic neoplasms (MCNs) and intraductal papillary mucinous neoplasms (IPMNs).
Methods: Using our prospective hospital EUS and surgical databases, we identified all patients who underwent EUS of a pancreatic cyst prior to surgical resection, in the last 10 years. Cysts were pathologically sub-classified as MCNs or IPMNs; all other cysts were considered non-mucinous. Values of cyst fluid CEA and amylase were correlated to corresponding surgical histopathology and compared between the two groups.
Results: 134 patients underwent surgery for pancreatic cysts including 82 (63%) that also had preoperative EUS. EUSFNA was performed in 61/82 (74%) and cyst fluid analysis in 35/61 (57%) including CEA and amylase in 35 and 33 patients, respectively. Histopathology in these 35 cysts demonstrated nonmucinous cysts in 10 and mucinous cysts in 25 including: MCNs (n=9) and IPMNs (n=16). Cyst fluid CEA (p=0.19) and amylase (p=0.64) between all IPMNs and MCNs were similar. Between branched duct IPMNs and MCNs alone, cyst fluid CEA (p=0.34) and amylase (p=0.92) were also similar.
Conclusion: In this single center study, pancreatic cyst fluid amylase and CEA levels appeared to be of limited value to influence the differential of mucinous pancreatic cysts. Larger studies are recommended to evaluate this role further.
Key words
pancreatic cysts; EUS; FNA; amylase; CEA
J Gastrointest Oncol 2011; 2: 208-214. DOI: 10.3978/j.issn.2078-6891.2011.020
|
|
Introduction
Mucinous pancreatic cysts are premalignant or malignant
pancreatic neoplasms. They usually are asymptomatic and
increasingly found due to widespread use of cross-sectional
abdominal imaging (CT scan and MRI). Radiologic features of mucinous cysts are often not distinguishable
from pseudocysts (PCs) or other cystic neoplasms with
minimal malignant potential such as serous cystadenomas
(SCAs) (1).
Mucinous pancreatic cysts are classified as mucinous
cystic neoplasms (MCNs with or without carcinoma) and
intraductal papillary mucinous neoplasms (IPMNs). The
latter are further classified into whether the neoplasm
involves the main pancreatic duct alone (main duct IPMN),
main pancreatic duct side branches alone (branched
IPMN), or both the main pancreatic and its side branches
(mixed IPMN). The grade of dysplasia in mucinous
pancreatic cysts is further classified as low grade dysplasia,
high grade dysplasia or invasive carcinoma (2).
Endoscopic ultrasound (EUS)-guided fine needle
aspiration (EUS-FNA) cytology with cyst fluid analysis is frequently utilized to aid in classification of pancreatic
cysts. However, the value of cytology is limited by the
frequently low cellularity of aspirated fluid (1). The utility of
several cyst fluid tumor markers studied has been variable
(3). Brugge et al. concluded that a cyst fluid CEA level of
192 ng/ml has the greatest area under the curve (AUC) for
differentiating mucinous from nonmucinous cysts (4). In a
pooled analysis of twelve studies, amylase <250 U/L from
cyst fluid was found to virtually exclude a pseudocyst. The
same study concluded that a CEA of <5 ng/ml and a CEA
>800ng/ml was strongly suggestive of a nonmucinous cyst
and mucinous cyst, respectively (5).
Combining clinical presentation with EUS morphology
and cyst fluid CEA concentration enhances the sensitivity
of differentiating mucinous from nonmucinous cysts (4).
However, planning appropriate management strategy often
requires further classifi cation of various types of mucinous
cysts (MCNs vs. IPMNs), particularly in asymptomatic
individuals with an increased surgical risk. For example,
surgical resection of all MCNs and main duct IPMNs in
surgically fit patients is recommended due to a significant
risk of malignant transformation. However, there is
increasing evidence that branched-duct IPMNs (BDIPMNs),
which are typically found in elderly individuals,
have less potential risk of malignancy. Therefore these
tumors are often monitored with surveillance imaging
without the need for surgical intervention (6,7).
It is not currently known whether pancreatic cyst fluid
markers can reliably distinguish between the various
subtypes of mucinous pancreatic cysts. The aim of the
current study is to determine whether pancreatic cyst fluid
CEA and amylase concentrations obtained by EUS-FNA
can diff erentiate either: 1) MCNs from IPMNs or; 2) MCNs
from BD-IPMNs.
|
|
Materials and Methods
Study population
This study was approved by the Institutional Review Board
of Indiana University Medical Center/Clarian Health
Partners. Using our prospectively maintained hospital
EUS and surgical databases, consecutive patients who
underwent EUS prior to surgical resection of a pancreatic
cyst over a 10 year period were identified. Hospital records,
endoscopy, histopathology, and surgical reports of these
patients were reviewed retrospectively. The following
clinical information was abstracted: age, gender and
symptoms. EUS features of pancreatic cysts noted included
the location (head, body, tail, multifocal), number and
size of the cysts, communication with the main pancreatic
duct or side branch, mural nodules, presence of septation, any associated solid mass. A dilated main pancreatic duct
was defined as greater than 3 mm, 2 mm, and 1 mm in the
head, body and tail, respectively. EUS-FNA puncture site,
number of passes, needle size, cytology results, and cyst
fluid carcinoembryonic antigen (CEA), and amylase were
noted. The type of surgery and final surgical histopathology
findings were also recorded.
Endoscopic ultrasound examination
After written informed consent was obtained, patients
received moderate or deep sedation using var ious
combinations of intravenous midazolam, meperidine,
fentanyl, or propofol under appropriate cardiorespiratory
monitoring. In accordance with a hospital-approved deep
sedation policy, registered nurse-administered propofol
sedation (NAPS) was available in our endoscopy for all
patients beginning in 2001 (8). During the second half
of the study period, commencement of deep sedation
was usually initiated with a combination of midazolam
and meperidine or fentanyl in order to minimize total
requirements of propofol (9). The choice of moderate
or deep sedation was made at the discretion of the
endosonographer. All procedures were performed by or
under the supervision of one of six experienced attending
endosonographers. EUS examinations were usually initiated
with an Olympus GF-UM20, GFUM-130 or GF-UM160
radial echoendoscope (Olympus America, Inc., Center
Valley, PA, USA). Curvilinear array endosonography was
performed using the Pentax 32-UA, Pentax 36-UX (Pentax
Medical Co, Montvale, NJ, USA), Olympus GF-UC30P,
or Olympus GF-UC140P-AL5 (Olympus America, Inc.,
Center Valley, PA, USA) echoendoscope. EUS-FNA was
generally performed only if the cyst size was ≥10 mm and if
the endosonographer believed that information gained from
cyst fluid analysis would impact patient management. FNA
was obtained using a 22-gauge EUSN-1, EUSN-2, EUSN-3,
or Echotip Ultra needle (Cook Medical Inc., Winston-
Salem, NC, USA) or EZ-Shot needle (Olympus America,
Inc., Center Valley, PA, USA). Doppler examination
was used to ensure the absence of intervening vascular
structures along the anticipated needle path. Depending on
the amount of blood anticipated during tissue sampling, full
or partial suction was applied. In general, a single EUS-FNA
pass was performed from the cyst but was repeated if the
endosonographer felt that further sampling would increase
the yield. Samples aspirated were expressed onto a glass
slide and two smear preparations were made. One slide was
air-dried and stained with a modified Giemsa stain for rapid
on-site interpretation, while the other slide was alcohol-fixed
and stained by the Papanicolaou method. A cytopathologist
was available on-site for preliminary diagnostic interpretations and assessment of specimen adequacy on all
procedures. If at least 1 ml of fluid was obtained from the
aspirate, analysis for carcinoembryonic antigen (CEA) and
amylase was requested. Definitive cytopathologic diagnoses
were given only after complete staining and subsequent
final interpretation was provided. One dose of intravenous
antibiotics (i.e. ampicillin/sulbactam or a fluoroquinolone)
was given immediately following the procedure followed
by 3-5 days of oral antibiotics (i.e. amoxicillin/clavulanate
or a fluoroquinolone) if EUS-FNA was performed. Per
department policy, all patients were telephoned within
48 hours after the procedure to assess for any short-term
complications.
Surgery and surgical pathology
All surgical consultations and operations were performed
by 1 of 5 experienced pancreatobiliary surgeons. Decisions
for surgery were based on a preoperative evaluation of the
patient’s fitness for operation coupled with the results of
all preoperative imaging studies. All patients had complete
abdominal exploration by laparoscopy or laparotomy to
rule out metastatic or locally advanced disease. A standard
pancreaticoduodenectomy or pylorus-preserving variant
was done for lesions located in the head or uncinate process.
A distal pancreatectomy and/or splenectomy were done
for tumors located in the body or tail. When tumors are
resected; routine intraoperative histologic frozen section
examinations were done on the pancreatic, bile duct, and
retroperitoneal soft tissue margins. A positive pancreatic or
bile duct margin for malignancy mandated further resection
until a negative margin was obtained. Persistently positive
pancreatic margins for malignancy or main duct IPMN
oft en resulted in a total pancreatectomy at the discretion of
the surgeon. Regional lymph nodes routinely resected en
bloc with the tumor specimen.
The final diagnosis in each patient was made by the result
of surgical resection and corresponding histopathology.
Histologic interpretation of resected specimens was
carried out by experienced gastrointestinal pathologists
and interpretation of the cystic lesions was made according
to WHO tumor classification as follows: “(1) a mucinous
cystic neoplasm (Low Grade Dysplasia (LGD), High Grade
Dysplasia (HGD), or malignant) or (2) a nonmucinous
cystic lesion including serous, inflammatory, and endocrine.
Cystic lesions arising from an intraductal papillary
mucinous tumor (IPMN) were considered mucinous” (10).
IPMNs were further classified as branched cysts only
(BD-IPMN) or involving the main pancreatic duct with
or without side-branched cysts (MD-IPMN). Malignant
mucinous cysts demonstrated were defined as the presence
of invasive carcinoma; all other neoplasms (including high grade dysplasia) were considered benign.
Statistical analysis
Continuous variables associations were assessed with
an unpaired t test. The association between categorical
variables of mucinous and non mucinous cysts was assessed
with the Fisher’s exact test. Mean values of cyst fluid CEA
(normal range 0-2.5 ng/ml) and amylase (normal range
25-115 U/L) were correlated to corresponding surgical
histopathology and compared using the Mann-Whitney
Test. A p-value less than 0.05 was considered statistically
significant.
|
|
Results
During the study period, 134 patients underwent
surgery for pancreatic cysts including: 87 (65%) classic
or pylorus sparing pancreaticoduodenectomies, 44
(33%) distal pancreatectomies and 3 (2%) total/subtotal
pancreatectomies. Of these, 82 (61%) patients (28
male; median age 60 years; range; 20-83) patients had a
preoperative EUS and comprised the study population
(Figure 1). No EUS-related complications were noted in any
patient.
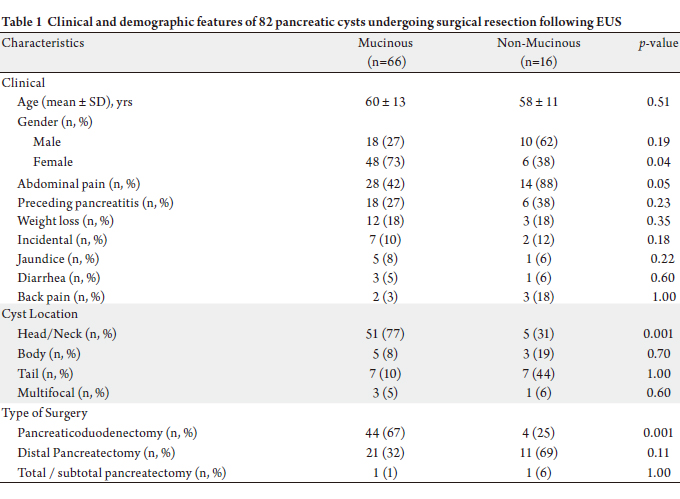
Surgical pathology revealed 66 mucinous and 16 nonmucinous
pancreatic cysts (Table 1). Although age was
similar between the two groups (p=0.51), mucinous cysts
were significantly more common in females (p=0.04).
No statistically significant difference in any presenting
symptom was noted between the two groups. Abdominal
pain was the most common presenting symptom (n=42, 58%), followed by preceding history of pancreatitis (n=24,
33%). Nine patients (7 mucinous and 2 non-mucinous) were
asymptomatic with a cyst found incidentally on abdominal
cross-sectional imaging done for other indications.
Mucinous tumors originated more often from pancreas
head and neck (p=0.001), and therefore were more likely to
require pancreaticoduodenectomy (P=0.001).
Final pathology from the 66 resected mucinous cysts
undergoing preoperative EUS included 14 MCNs and
52 IPMNs. None of the MCNs had high grade dysplasia
(HGD) or cancer. Pathology from the 52 IPMNs included:
low grade dysplasia (LGD) in 37, HGD in 7, and invasive
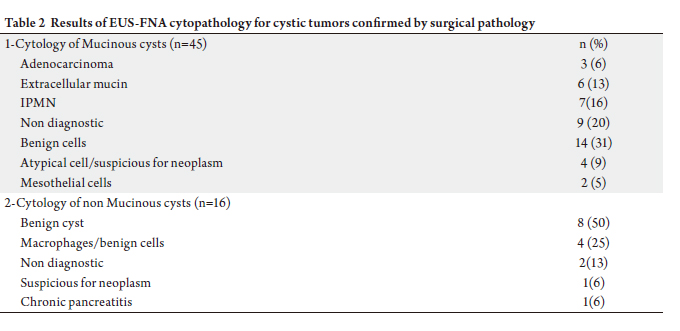
cancer in 8. EUS-FNA (Table 2) was performed in 61/82
(74%), including 16 of 16 (100%) of the non-mucinous cysts
and 45 of 66 (68%) mucinous tumors. Cyst fluid analysis
was feasible in 35/61 (57%) patients, including CEA and
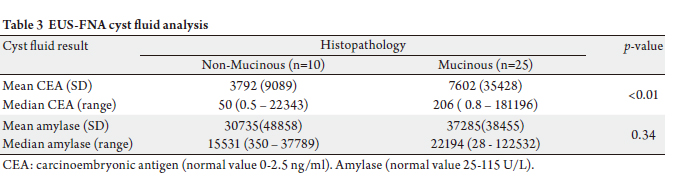
amylase levels in 35 and 33 patients, respectively (Table 3).
Histopathology in these 35 pancreatic cysts demonstrated
10 non-mucinous cysts and 25 mucinous cysts.
The 10 non-mucinous cysts included 7 serous
cystadenomas (mean CEA = 81 ng/ml, median CEA = 92
ng/ml; range: 0.5 – 310 ng/ml; mean amylase = 3209 U/L,
median amylase = 1111 U/L; range: 350-146702 U/L) and
3 pseudocysts (mean CEA = 177 ng/ml, median CEA = 93; range 1.7-410 ng/ml; mean amylase = 28610 U/L, median
amylase 28208 U/L; range 19834-37789 U/L).
The 25 mucinous cysts included 9 MCNs (mean CEA
= 21119 ng/ml, median CEA 813 ng/ml; range 1.3-181196
ng/ml; mean amylase = 45567 U/L, median amylase =
41153 U/L; range 28-102400), 11 IPMN-Br (including one
cancer and 5 HGD; mean CEA = 613 ng/ml, median CEA
= 426 ng/ml; range 3.8-4878 ng/ml, mean amylase = 25641
U/L, median amylase = 744 U/L; range 223- 53200 U/L)
and 5 IPMN-M (including one cancer; mean CEA = 143
ng/ml, median CEA 181 ng/ml; range 43-298 ng/ml, mean
amylase = 67763 U/L, median = amylase 14580 U/L; range
744-108451 U/L).
Mean CEAs were greater for mucinous compared to nonmucinous
cysts, however there was no statistically significant
difference in cyst fluid amylase levels between the two groups
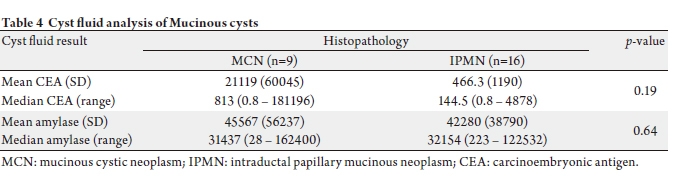
(Table 3). Comparison between cyst fluid CEA and amylase
for all 25 mucinous cysts are shown in the Table 4. As shown,
cyst fluid CEA (p=0.34) and amylase (p=0.92) were also
similar between BD-IPMNs and MCNs alone.
    |
|
Discussion
Pancreatic cysts are increasingly detected due to widespread use of cross sectional imaging like CT scan
and MRI. The majority of pancreatic cystic lesions are
benign such as pseudocysts and serous cystadenomas.
However, it is estimated that 10-15% of pancreatic cysts are
potentially premalignant or malignant cystic neoplasms,
(usually mucinous cysts) that require further evaluation,
management and follow up (1,11). EUS has emerged as
the preferred modality to study these lesions because it
provides high resolution images and morphologic detail
compared to other imaging techniques. EUS-FNA also
permits collection of cyst fluid for analysis for diagnostic
markers such as CEA, CA19-9, CA 72-4, CA-125, amylase, and lipase to help differentiate among different types of
pancreatic cysts (12). A cyst fluid CEA of 192 ng/ml appears
to optimize the diagnosis of mucinous with non-mucinous
tumors (4). However, it is not known whether pancreatic
cyst fluid markers can reliably differentiate one type of
mucinous pancreatic cyst from another.
In the present study, we performed a cohort analysis of
cyst fluid markers in patients who underwent EUS-FNA
prior to surgery to investigate whether cyst CEA and/or
amylase levels would aid in the differential diagnosis of
various types of mucinous cysts. Sixty-six of the 82 (80%)
patients in the study population who underwent surgery had pathologically confirmed mucinous lesions and a variant
of IPMN were found in 52 (63%). Clinical symptoms at
presentation did not vary significantly between mucinous
and non-mucinous cysts and similar to prior reports,
females were more commonly found to have mucinous
compared to nonmucinous cysts (2,13).
Cyst fluid analysis was feasible in 43% of our cohort.
Similar to previous reports, we found that cyst fluid CEA
was significantly higher in mucinous compared to nonmucinous
lesions. However, amylase was similar between
the two groups (p=0.34). Amylase is reportedly elevated
in cyst fluid that communicates with the pancreatic ductal
system, such as pseudocysts and IPMNs. However, cyst
fluid amylase is not typically elevated in tumors with only
rare ductal communication such as SCAs or MCNs (14,15).
Since most mucinous cysts in our series are of the IPMN
type, a significant overlap in the amylase value could
explains the lack of differentiation of this marker among
various cyst types.
We also found that cyst amylase and CEA are similar
among BD–IPMNs and MCNs. This is clinically relevant
since these two types of mucinous cysts with normal
diameter main pancreatic ducts may be difficult to
differentiate by morphologic imaging alone. Current
guidelines recommend surgical resection for MCNs but
recent data suggest that BD-IPMN smaller than 3 cm
without referable symptoms or recent enlargement may
be followed clinically (16). Our data suggest that cyst fluid
CEA and amylase cannot be used to distinguish these two
groups. Prior smaller studies have shown variable results
(17-19). Khalid et al. have shown that DNA analysis can
point to a mucinous lesion when there is uncertainty from
the CEA analysis alone. However, the same study has not
proven that DNA analysis can help distinguish BD-IPMN
from MCNs (3).
The current series is an additional demonstration of the
clinical challenge to accurately predict cyst pathology in
order to plan proper patient management. While there is
an increasing interest in non-surgical management of many
neoplastic cysts (20,21), the precise preoperative diagnosis
is crucial (6,22). Recently developed molecular analyses
of cyst fluid may provide a promising role in distinction of
nonmucinous from mucinous cyst in general and of benign
and malignant cysts in particular (3,23).
This series has a few limitations that merit discussion.
This is a retrospective study from a single tertiary referral
center and thus could have been underpowered to detect
a true difference in CEA levels between the two cyst types
studied. Only 61% of all resected pancreatic cysts during
the study period had preoperative EUS evaluation, and
more than half of the cases did not undergo EUS-FNA. This could be explained by the fact that the decision to
resect many of the pancreatic cysts especially the large
ones which may have been symptomatic then was based
on conventional imaging features as well as the clinical
presentation; therefor EUS with fluid aspiration results may
not have felt to influence the treatment course and therefore
were not referred for preoperative EUS evaluation. Of those
who underwent FNA, cyst fluid analysis was technically
not feasible in about one-third of patients, due to technical
reasons or small fluid volume amenable for adequate
laboratory testing. Thus, type I error and referral bias is
expected since most surgeries in this series were performed
for malignant or highly suspicious premalignant lesions.
In conclusion, the current series suggest that pancreatic
cyst fluid amylase and CEA levels may not appear to
distinguish BD-IPMNs from MCNs. However; larger
adequately powered studies are needed to evaluate
this further. Therefore, clinical picture, cyst imaging
morphology and evaluation of the presence (IPMN) or
absence (MCN) of pancreatic duct communication remains
the up-to-date tools to differentiate these two groups.
|
|
Conflict of Interest
Th e authors have no conflict of interest to declare related to
this work.
|
|
References
Cite this article as:
Al-Rashdan A, Schmidt C, Al-Haddad M, McHenry L, LeBlanc J, Sherman S, Dewitt J. Fluid analysis prior to surgical resection of suspected mucinous
pancreatic cysts. A single centre experience. J Gastrointest Oncol. 2011;2(4):208-214. DOI:10.3978/j.issn.2078-6891.2011.020
|