Single institution experience of sorafenib for advanced HCC in a US tertiary care hospital
Introduction
Sorafenib is an orally administered small molecule tyrosine kinase inhibitor that has anti-angiogenic and pro-apoptotic effects (1,2). It’s the first-line systemic therapy for advanced hepatocellular carcinoma (HCC) approved by U.S. Food and Drug Administration (3). Two phase III randomized controlled trials showed benefit of overall survival with sorafenib in patients with advanced HCC and Child-Turcotte-Pugh (CTP) class A liver function (4,5). A phase II trial proved its safety in CTP class B cirrhosis (6). Many observational studies, including the Global investigation of therapeutic decisions in HCC and of its treatment with sorafenib (GIDEON) study have also demonstrated the efficacy of sorafenib in advanced HCC (7). However, a recent study on Medicare data has questioned the applicability of the former phase III trials to a US population (8).
There has also been interest in identifying prognostic and predictive factors for survival in patients with advanced HCC treated with sorafenib. A post hoc sub group analysis of the phase III trials identified absence of extrahepatic spread (EHS), hepatitis C virus (HCV) infection and low neutrophil-to-lymphocyte (NLR) ratio as predictors of greater overall survival benefit (9). Observational studies done in Europe and South-East Asia have found a variety of variables to be of predictive and prognostic value (10-13). In the present study done at an inner-city safety net hospital of Chicago, we aimed to evaluate the adverse effect profile, outcomes and identify predictive and prognostic factors for survival associated with use of sorafenib in HCC.
Methods
We retrospectively reviewed the electronic medical records of adult patients (age >18 years) with HCC who presented to John H. Stroger Hospital of Cook County, Chicago, IL, from January 01, 2009 through July 31, 2015. We identified potential patients using ICD-9 code (= 155) and/or ICD-10 code (= C22) for malignant neoplasm of liver and intrahepatic biliary duct. We confirmed the histopathological or imaging diagnosis (intense enhancement during arterial phase followed by “washout patter” during delayed phase, seen on triple phase CT or magnetic resonance imaging in patients with cirrhosis) and included patients who received sorafenib in our analysis. Patients enrolled in clinical trials, who received oncologic care in another hospital or those who received additional systemic chemotherapeutic agents were excluded. The present study was approved by the Institutional Review Board of Cook County Health & Hospitals System, Chicago. The database was set up and maintained by the Department of Medicine, Cook County Health & Hospitals System.
Sorafenib dosing
Sorafenib was started at daily dose of 800 mg in all patients. As per the discretion of the treating medical oncologist, it was discontinued with the development of an adverse event or progression of disease. In some patients, the dose was decreased if the daily 800 mg dose was not tolerated.
Data collection
Eligible patients were censored if there was discontinuation of follow up. We collected data pertaining to patient demographics, tumor, management plans and laboratory investigations, recorded at the time of sorafenib initiation. These include age, gender, race, date of death, etiology of liver disease, CTP class, presence of EHS, portal vein invasion, Barcelona stage of HCC, previous locoregional therapy, duration of sorafenib therapy, adverse events recorded by the treating physician and laboratory values [neutrophil, lymphocyte, hemoglobin, platelet, international normalized ratio (INR), albumin, aspartate aminotransferase, total bilirubin]. EHS was defined as the presence of metastases to abdominal lymph nodes or other solid organs. Portal vein invasion was defined as evidence of it seen on any form of imaging.
Data analysis
Descriptive data was summarized using mean and percentages for continuous and categorical variables, respectively. Overall survival was presented as median with interquartile range. It was calculated as the time from sorafenib initiation to death. Patients with treatment duration greater than 1 month were included in the Kaplan-Meier survival comparisons, to identify potential predictive and prognostic factors. Log rank test was used to compare survival curves. All assumptions of Kaplan-Meier curves were met. Chi square test and Fischer’s exact test were used to compare frequency of categorical variables and find associations. Student’s t test was used to compare means of two groups. All assumptions for Chi-square and Student’s t test were met. P value of lesser than or equal to 0.05 was considered to statistically significant. SPSS 21 (IBM Corp. Released 2012. Version 21.0. Armonk, NY, USA) was used for the data analysis.
Results
Fifty-nine patients received sorafenib in the study period. Nineteen of them died during the follow up period. Mean age of the population was 57.6 years (standard deviation 8.4 years) and 81% of them were male. Predominant etiologies of liver disease in the cohort were alcohol (63%), HCV infection (46%) and hepatitis B virus (HBV) infection (25%). Sixty-four percent of them had CTP class A cirrhosis or had no cirrhosis. Most common Barcelona stage of the tumor was stage C (73%) and EHS and portal vein invasion were seen in 34% and 41% of the patients, respectively. Descriptive data pertaining to the baseline characteristics of the patients included in the cohort are summarized in Table 1.
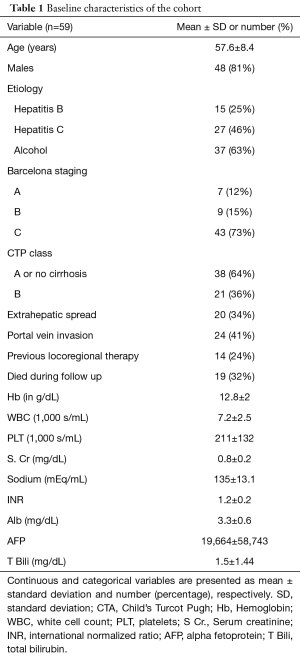
Full table
Safety
Twenty-seven of the patients developed adverse effects with sorafenib. It was discontinued in nineteen of them. Median duration of sorafenib treatment in entire cohort was 4.6 months. Most common adverse effects were hand foot syndrome [15] and diarrhea [13]. These are summarized in Table 2.

Full table
Outcomes
Median overall survival was 7 months (25–75 percentile =3–15 months). Patients who had received sorafenib for more than 1 month (n=48) were included into the Kaplan-Meier analysis. Patients with CTP class A cirrhosis or no cirrhosis (median OS 39 vs. 16 months, log rank test 3.913, P=0.048), HCV infection (median OS 39 vs. 9 months, log rank test 5.015, P=0.025) and absence of EHS (median 39 vs. 9 months, log rank test 5.632, P=0.018) had better overall survival. Their respective Kaplan-Meier curves are shown in Figure 1A,B,C. Comparison of baseline characteristics of patients with and without HCV infection is shown in Table 3. Presence of portal vein invasion (P=0.602), previous locoregional therapy (P=0.084), alcoholic liver disease (P=0.296), HBV infection (P=0.378) and history of adverse effects with sorafenib (P=0.69) did not affect overall survival.
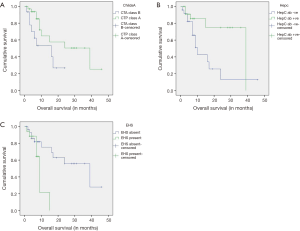
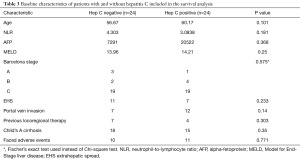
Full table
Discussion
To the best of our knowledge, this is the first US single institution experience of sorafenib in advanced HCC. The GIDEON study is the only other published study which describes the real-world experience with sorafenib in a US population (7). Fifty-nine patients were treated with sorafenib for HCC over a span of 6 years in our institution. As the study site was a safety-net hospital serving an underserved population and immigrants, there was a greater proportion of patients with HBV infection (25% vs. 14%) and alcohol related liver disease (63% vs. 39%) compared to the US arm of GIDEON study. The median duration of treatment was comparable to that of the US arm of GIDEON study (4 vs. 4.6 months).
The median survival of our population was slightly lower than of the US arm of GIDEON study (7 vs. 8.5 months) (7). A study based on Surveillance, Epidemiology and End Results Program and Medicaid claim data showed a median survival of 3 months from the first prescription of sorafenib in Medicaid patients with advanced HCC in US patients (8). However, it used indirect data sources which could be prone for various forms of bias, making it less reliable. Real world experiences in Europe (14-18) and Japan (19) estimated the median overall survival of patients with advanced HCC after sorafenib initiation to be 10–15 months. Even in the GIDEON study (7), the median overall survival of US patients was lower than Europe and Japan. Interestingly, a prospective multi-center study in Japan has estimated median overall survival of 10.1 months despite higher proportion of patients with EHS and Barcelona stage C (19) than the presented cohort. Even though a head to head comparison of the overall survival from different cohort can’t be made due to differences in baseline characteristics of patients, it raises the possibility of social or unknown medical or epidemiologic variables affecting survival of patients receiving sorafenib for HCC in US. The pertinent characteristics of the above-mentioned studies along with the median overall survival are shown in Table 4.

Full table
The adverse effect profile and frequency were similar to those seen in phase III trials and real-world experiences (4,5,15-17). In our study, patients with CTP class A cirrhosis and those without EHS had better survival with sorafenib. This has been replicated in various studies (9,15,16,18) and can be explained by the fact that liver function and extent of cancer spread are established prognostic indicators in all HCC patients (20). Interestingly, HCV infected patients had better survival than those not infected with HCV. A review of the baseline characteristics of these two groups (as shown in Table 3) shows that the two comparison groups were largely similar, decreasing the likelihood of confounding. This association was also noted in a post hoc exploratory sub group analysis of the landmark phase III trials for sorafenib in advanced HCC (9). There is evidence to suggest that sorafenib doesn’t affect HCV replication in vivo (21) and exact pathophysiologic mechanisms of this association are unknown. Sorafenib inhibits multiple tyrosine kinase pathways and also has additional mechanisms independent of tyrosine kinase inhibition (22). It’s preferential effect in patients with HCV infection could be due to specific biochemical or biological actions unique to HCV induced carcinogenesis or HCV related HCC metastases. Understanding these mechanisms could be of future significance as it could lead to the development of effective personalized therapies useful for this subset of patients with HCC.
Our study has few limitations. Firstly, it was a retrospective study and is prone to various biases (23), which include absence of control group and possible confounding through unmeasured variables. Secondly, the small sample size may hamper its external validity. We could not perform Cox Regression analysis and due to the limited effective sample size of nineteen, which is equal to the number of deaths recorded in a survival analysis (24). We also could not run subgroup analysis in hepatitis C patients based on viral load or genotype due to the above reason. Thirdly, we did not have information on cause of deaths of the patients.
Conclusions
The median overall survival benefit in patients receiving sorafenib for advanced HCC is probably lesser in US population than in Europe and Japan, cause of which requires investigation. It’s relatively safe and its common adverse effects are diarrhea and hand-foot syndrome. HCV infection could be a predictive factor in patients receiving sorafenib for advanced HCC.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The present study was approved by the Institutional Review Board of Cook County Health & Hospitals System, Chicago (No. 16-024).
References
- Chang YS, Adnane J, Trail PA, et al. Sorafenib (BAY 43-9006) inhibits tumor growth and vascularization and induces tumor apoptosis and hypoxia in RCC xenograft models. Cancer Chemother Pharmacol 2007;59:561-74. [Crossref] [PubMed]
- Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 Exhibits Broad Spectrum Oral Antitumor Activity and Targets the RAF/MEK/ERK Pathway and Receptor Tyrosine Kinases Involved in Tumor Progression and Angiogenesis. Cancer Res 2004;64:7099-109. [Crossref] [PubMed]
- Kane RC, Farrell AT, Madabushi R, et al. Sorafenib for the treatment of unresectable hepatocellular carcinoma. Oncologist 2009;14:95-100. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in Advanced Hepatocellular Carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 2006;24:4293-300. [Crossref] [PubMed]
- Cho JY, Paik YH, Lim HY, et al. Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma. Liver Int 2013;33:950-7. [Crossref] [PubMed]
- Lee S, Kim BK, Kim SU, et al. Clinical outcomes and prognostic factors of patients with advanced hepatocellular carcinoma treated with sorafenib as first-line therapy: a Korean multicenter study. J Gastroenterol Hepatol 2014;29:1463-9. [Crossref] [PubMed]
- Ozenne V, Paradis V, Pernot S, et al. Tolerance and outcome of patients with unresectable hepatocellular carcinoma treated with sorafenib. Eur J Gastroenterol Hepatol 2010;22:1106-10. [Crossref] [PubMed]
- Takeda H, Nishikawa H, Osaki Y, et al. Clinical features associated with radiological response to sorafenib in unresectable hepatocellular carcinoma: a large multicenter study in Japan. Liver Int 2015;35:1581-9. [Crossref] [PubMed]
- Sanoff HK, Chang Y, Lund JL, et al. Sorafenib Effectiveness in Advanced Hepatocellular Carcinoma. Oncologist 2016;21:1113-20. [Crossref] [PubMed]
- Kudo M, Lencioni R, Marrero JA, et al. Regional differences in sorafenib-treated patients with hepatocellular carcinoma: GIDEON observational study. Liver Int 2016;36:1196-205. [Crossref] [PubMed]
- Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol 2017;67:999-1008. [Crossref] [PubMed]
- Di Costanzo GG, Tortora R, Morisco F, et al. Impact of Diabetes on Outcomes of Sorafenib Therapy for Hepatocellular Carcinoma. Target Oncol 2017;12:61-7. [Crossref] [PubMed]
- Ganten TM, Stauber RE, Schott E, et al. Sorafenib in Patients with Hepatocellular Carcinoma-Results of the Observational INSIGHT Study. Clin Cancer Res 2017;23:5720-8. [Crossref] [PubMed]
- Hollebecque A, Cattan S, Romano O, et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: the impact of the Child-Pugh score. Aliment Pharmacol Ther 2011;34:1193-201. [Crossref] [PubMed]
- Iavarone M, Cabibbo G, Piscaglia F, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology 2011;54:2055-63. [Crossref] [PubMed]
- Pressiani T, Boni C, Rimassa L, et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: a prospective feasibility analysis. Ann Oncol 2013;24:406-11. [Crossref] [PubMed]
- Nakano M, Tanaka M, Kuromatsu R, et al. Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Med 2015;4:1836-43. [Crossref] [PubMed]
- Kim DY, Kim HJ, Han KH, et al. Real-Life Experience of Sorafenib Treatment for Hepatocellular Carcinoma in Korea: From GIDEON Data. Cancer Res Treat 2016;48:1243-52. [Crossref] [PubMed]
- Tandon P, Garcia-Tsao G. Prognostic indicators in hepatocellular carcinoma: a systematic review of 72 studies. Liver Int 2009;29:502-10. [Crossref] [PubMed]
- Cabrera R, Limaye AR, Horne P, et al. The antiviral effect of sorafenib in hepatitis c-related hepatocellular carcinoma. Aliment Pharmacol Ther 2013;37:91-7. [Crossref] [PubMed]
- Tofthagen C. Threats to Validity in Retrospective Studies. J Adv Pract Oncol 2012;3:181-3. [PubMed]
- Babyak MA. What you see may not be what you get: a brief, nontechnical introduction to overfitting in regression-type models. Psychosom Med 2004;66:411-21. [PubMed]


