
Gastric adenocarcinoma revealed by atypical pulmonary lymphangitic carcinomatosis
Introduction
Gastric cancer most frequently presents with symptoms of weight loss (62%), abdominal pain (52%), nausea (34%), dysphagia (26%) and melaena (20%) (1). Dyspnea is a very rare presentation of gastric cancer (2). Apart from lung metastases, two other aetiologies can account for this presentation: pulmonary lymphangitic carcinomatosis (PLC) or pulmonary tumor thrombotic microangiopathy (PTTM).
We report the unusual case of a 53-year-old woman with progressive dyspnea and a pulmonary interstitial disease leading to a diagnosis of gastric carcinoma. The atypical radiology appearance was suggestive for both PLC and PTTM. We discuss the pathological mechanisms based on current literature.
Case presentation
History and clinical examination
A 53-year-old Chinese woman was admitted in the emergency department with a 2-month history of persistent cough. The cough was productive with clear phlegm mostly at night, sometimes associated with vomiting and improved by antacid medication. She also described worsening breathlessness over 2 months weight loss (4 kg) and occasional night sweats and fever. She had no prior history of tuberculosis, had not travelled recently nor was exposed to any respiratory irritants. Her past medical history included hypertension and well controlled diabetes. Her medication included amlodipine and metformin and no new medicines had been started recently. She was working as an administrative agent. There was no history of smoking or alcoholism.
Clinical examination revealed tachypnea with a respiratory rate of 25 breaths/min and reduced oxygen saturation on pulse oxymetry 91% at rest, falling to 86% on minimal exertion (after clinical examination). Bilateral basal lung crepitations were noted on auscultation. She was hemodynamically stable with no other abnormalities identified.
Biochemical investigations
Laboratory investigations showed a raised CRP at 33 mg/L (<5 mg/L) and a strongly positive CA 19-9 at 2,872 kU/L [0–37]. Detailed investigation results are shown in Table 1.
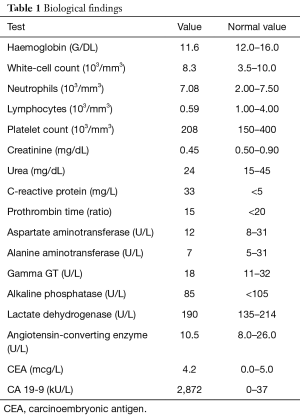
Full table
Imaging
Chest X-ray showed diffused parenchymal infiltration.
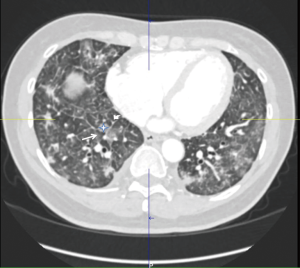
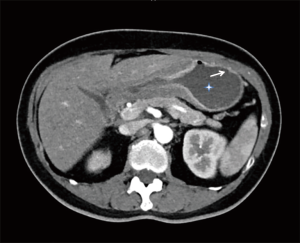
High resolution computed tomography (HRCT) (thoracic and abdominal): (Figure 1) numerous bilateral small nodules around 1 cm in diameter. Ground glass opacities in combination with enlarged interlobular septa giving a “crazy paving pattern” appearance were present bilaterally. Pleural thickening was also noted on the right base with a small pleural effusion. There was an enlargement of the pulmonary artery at 33 millimetres but no evidence of pulmonary emboli. The abdominal CT showed thickening of the gastric antrum associated with enlarged lymph nodes and a peritoneal carcinomatosis (Figure 2).
Echocardiography: pulmonary arterial hypertension was noted at 39 mmHg + central venous pressure.
Bronchoscopy was normal. Bronchoalveolar lavage was consistent with non-specific inflammation (30% neutrophils). No specific lesion was identified. Trans-bronchial biopsies revealed a few malignant cells of gastro-intestinal origin. Microbiology including mycobacteria, viruses was negative.
Gastroscopy revealed an antral ulcer. Biopsy consistent with a cytokeratin 7, 20 and 2 positive gastric adenocarcinoma Helicobacter pylori testing was negative.
PET scan increased gastric uptake and infiltration in both lungs with a higher apical uptake.
The patient decided to return to her home country so no further treatment or follow up was done at our hospital.
Discussion
PLC and PTTM are both strongly associated with gastric cancer (1) but are very uncommon presentations. To our knowledge, the association of these two vascular metastatic presentations in one patient is not reported based on the atypical appearance.
PLC is characterized by tumour invasion of pulmonary lymph nodes or the adjacent interstitial tissue, resulting in thickening of the bronchovascular bundle and septa. The main clinical manifestations are cough and dyspnoea. The most frequent malignancies associated with this pathological condition are breast cancer (33%), gastric cancer (29%), pancreatic cancer (17%), lung cancer (4%) and prostate cancer (3%) (3). Usual radiological findings on plain X-ray are a reticulonodular pattern, pleural effusion in 30% to 50% of cases and hilar or mediastinal lymphadenopathy in 20% to 40% of cases. However, up to 30% to 50% of patients with PLC can have a normal chest radiograph. Thickening of interlobular septa and peri-bronchovascular interstitium, as well as small size lymphadenopathy, are the most frequent findings on HRCT (2). PLC is associated with a poor prognosis with an average survival of 3 months (4).
PTTM is characterised by tumour cell invasion of the pulmonary vascular system with small tumour emboli found on histology. They are distinct from conventional tumour emboli: in PTTM fibrocellular and/or fibromuscular intimal proliferation leads to vascular stenosis that in turn triggers platelet aggregation and thrombosis (5,6). This diffuse thrombosis can lead to severe pulmonary hypertension (7,8). PTTM is significantly associated with gastric cancer compared to other types of cancers (9). The main clinical symptoms are dyspnoea and right heart failure due to severe pulmonary hypertension (10,11). Radiological findings are non-specific but a “tree in bud” pattern on HRCT is usual, thought to be due to fibrocellular intimal hyperplasia in small arteries and arterioles (12). Some coagulation indices may also be modified in this condition due to thrombotic events such as prolonged prothrombin and activated partial thromboplastin times, low fibrinogen levels and elevated D-dimers (13). Similar to PLC, prognosis is very poor with an average of 16.2 days. Diagnosis of PTTM is based on clinical findings as, given the rapidity of clinical deterioration, bronchoscopy and lung biopsy are often difficult to perform (14).
In this case, the HRCT scan revealed diffuse bilateral small nodules, ground glass opacities, thickening of interlobular septa and evidence of pulmonary hypertension. Ground glass appearance from interstitial oedema or extension of the tumour into the parenchyma is often seen in PLC (2). However it can also be found in infections, especially opportunistic infections such as pneumocystis jirovecii, cytomegalovirus or herpes simplex (15). Bronchoscopy and BAL in this patient did not reveal any evidence of infection so it is likely they were due to underlying PLC. Smooth (early stage) and nodular (late stage) thickening of interlobular septa and peribronchovascular interstitium are also commonly seen in PLC due to haematogenous metastasis (2).
The combination of interlobular thickening and ground glass opacities gives the characteristic “crazy paving pattern” seen in our case and also described in PLC. Other, more common, aetiologies of “crazy paving pattern” appearance are acute respiratory distress syndrome, pulmonary alveolar proteinosis or acute interstitial pneumonia (16,17). In addition to the above, interestingly our patient also showed evidence of pulmonary hypertension with an enlarged pulmonary artery at 33 mm and elevated pressures on echocardiography (39 mmHg + central venous pressure). It is possible that these appearances were due to idiopathic pulmonary hypertension, however, in view of the underlying pathology, PTTM, which can be accompanied by pulmonary hypertension secondary to vascular stenosis and thrombus formation (10,11), is also likely even though coagulation markers were not disturbed.
Summary of different elements comparing PTTM and PLC are shown on Table 2.
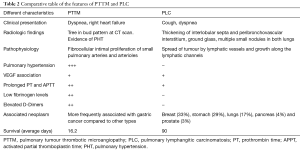
Full table
PLC and PTTM pathophysiology: the central role of VEGF
Pathophysiology of PLC and PTTM
The pathophysiology of PLC and PTTM was initially thought to be due to obstruction of the vessel lumen (lymphatic or vascular) by tumour cell invasion and subsequent secondary effects such as pulmonary hypertension. However, recently an additional pathophysiologic mechanism has also been proposed involving vascular endothelial growth factor (VEGF) and tissue factor (TF).
VEGF is a homodimeric glycoprotein with a molecular weight of approximately 45 kDa. It is a key mediator of angiogenesis (formation of new blood vessels), promoting new vessel formation during development, wound healing and the menstrual cycle in adults. It also plays an important role in cancer development: it is up-regulated by oncogenes, growth factors and hypoxia and promotes new, anarchic vessel formation in tumours thus promoting their survival. These abnormal blood vessels lead to early hypoxia and further VEGF production (18). With the evolution of molecular characterization, a number of VEGF isoforms have been identified along with their specific receptors. VEGF A regulates angiogenesis by binding on VEGF receptor 1 and 2 (VEGFR 1/2) localized on endothelial cells, whereas VEGF C and D promote lymphangiogenesis by binding to VEGFR 3, localized on lymphatic vessels (19). In addition to its role in angiogenesis and lymphangiogenesis, VEGF may affect haemostasis through increased expression of TF and thrombomodulin (TM) in endothelial cells, two molecules important for the initiation of the extrinsic and inhibition of coagulation pathways respectively (20-22).
High VEGF expression is characteristic of gastric cancer (23). VEGF is believed to be secreted by cancer cells (24) and then stored in platelets (alpha granules) and released in the serum upon platelet activation during clotting (25). The effects of VEGF are still not well understood in this context, however it is thought to play a central role in promoting tumour metastasis according to different mechanism.
It increases vascular permeability promoting tumour invasiveness (26)
Mirzapoiazova et al. (26) studied in 2006 the effect of VEGF on human pulmonary endothelial cells. They found that high VEGF levels caused disruption of adherens junctions leading to a higher permeability of blood vessels and increased cellular migration, both processes which would facilitate gastric cancer lung metastasis.
It stimulates cell migration responsible for haematogenous and lymphatic metastasis (27)
Das and colleagues reported that VEGF C expression by tumour cells in mouse models of tumour metastasis, induced lung lymphangiogenesis and promoted intralymphatic spread of metastasis and formation of tumour emboli in the pulmonary arteries, characteristic of PLC. In addition VEGF-C expression by tumour cells altered the pattern of pulmonary metastasis from nodular to diffuse and facilitated disease progression (27). Interestingly, Wang et al., verified this fact and showed that serum VEGF-C was higher in patients with lymph node metastasis and distant metastasis (28). Other studies supported this idea as Bilgiç et al. (29), showing that VEGF levels correlated with the presence of metastatic tissue. Ilhan et al. (30) showed a statistically significant increase of VEGF levels directly linked to the tumour stage (29,30).
It stimulates expression of TF and TM in endothelial cells (21,22)
Expression of TF on the surface of endothelial cells, upon contact with its circulating ligand (factor VII), activates the extrinsic coagulation cascade leading to thrombosis, a characteristic feature of PTTM in association with gastric cancer. TM, also expressed on endothelial cells, has a dual role in the coagulation cascade, both inhibiting coagulation through increased activation of protein-C and promoting thrombus formation through activation of thrombin-activatable fibrinolysis inhibitor (TAFI). VEGF has been shown to increase expression of both molecules (21,22) and although not directly demonstrated, one could speculate that the increased VEGF expression associated with gastric cancer could promote pulmonary vascular thrombus formation, characteristic of PTTM, via this mechanism.
It is therefore possible that VEGF, through its actions on endothelial cells and the coagulation system, is a common denominator in the pathogenesis of both PLC and PTTM. In a recent literature review, VEGF-A was identified as a useful biomarker for gastric cancer disease progression and remission but not for diagnosis (31). It would be interesting to determine whether serum levels of VEGF correlate with gastric cancer metastasis and development of PLC or PTTM as they could serve as an important early diagnostic tool. Apart from VEGF, novel markers like the long non-coding microRNA MALAT-1, recently shown to promote invasiveness of gastric cancer cells (32), could be examined in relation to their role in pulmonary invasiveness of gastric cancer. These new markers could help improve our understanding of the mechanism of gastric cancer metastasis and therefore the development of targeted treatments.
Conclusions
PLC as well as PTTM are two pulmonary manifestations of gastric cancer that clinicians have to recognize quickly in order to start treatment early as prognosis is poor. Details of the pathophysiology remain unclear. However a role for VEGF in both conditions is beginning to emerge. This along with novel molecules that could be important in the pathophysiology of pulmonary metastasis in gastric cancer could be new therapeutic targets in the future.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: The patient died a few days after returning to her home country. Her son gave written consent for the case report to be published.
References
- Wanebo HJ, Kennedy BJ, Chmiel J, et al. Cancer of the stomach. A patient care study by the American College of Surgeons. Ann Surg 1993;218:583-92. [Crossref] [PubMed]
- Desigan G, Wang M, Wofford B, et al. Occult gastric cancer manifested by progressive shortness of breath in a young adult. South Med J 1986;79:1173-6. [Crossref] [PubMed]
- Moubax K, Wuyts W, Vandecaveye V, et al. Pulmonary lymphangitic carcinomatosis as a primary manifestation of gastric carcinoma in a young adult: a case report and review of the literature. BMC Res Notes 2012;5:638. [Crossref] [PubMed]
- Yang SP, Lin CC. Lymphangitic carcinomatosis of the lungs. The clinical significance of its roentgenologic classification. Chest 1972;62:179-87. [Crossref] [PubMed]
- von Herbay A, Illes A, Waldherr R, et al. Pulmonary tumor thrombotic microangiopathy with pulmonary hypertension. Cancer 1990;66:587-92. [Crossref] [PubMed]
- Sato Y, Marutsuka K, Asada Y, et al. Pulmonary tumor thrombotic microangiopathy. Pathol Int 1995;45:436-40. [Crossref] [PubMed]
- Yao DX, Flieder DB, Hoda SA. Pulmonary tumor thrombotic microangiopathy: an often missed antemortem diagnosis. Arch Pathol Lab Med 2001;125:304-5. [PubMed]
- Chinen K, Kazumoto T, Ohkura Y, et al. Pulmonary tumor thrombotic microangiopathy caused by a gastric carcinoma expressing vascular endothelial growth factor and tissue factor. Pathol Int 2005;55:27-31. [Crossref] [PubMed]
- Fujishiro T, Shuto K, Shiratori T, et al. A case report of pulmonary tumor thrombotic microangiopathy (PTTM) caused by esophageal squamous cell carcinoma. Esophagus 2013;10:247-51. [Crossref] [PubMed]
- Shih HM, Lin CC, Shiao YW. Pulmonary tumor thrombotic microangiopathy. Am J Emerg Med 2011;29:241.e3-4. [Crossref] [PubMed]
- Keenan NG, Nicholson AG, Oldershaw PJ. Fatal acute pulmonary hypertension caused by pulmonary tumour thrombotic microangiopathy. Int J Cardiol 2008;124:e11-3. [Crossref] [PubMed]
- Franquet T, Gimenez A, Prats R, et al. Thrombotic microangiopathy of pulmonary tumors: a vascular cause of tree-in-bud pattern on CT. AJR Am J Roentgenol 2002;179:897-9. [Crossref] [PubMed]
- Mandaliya R, Farhat S, Uprety D, et al. Occult gastric cancer presenting as hypoxia from pulmonary tumor thrombotic microangiopathy. J Gastric Cancer 2014;14:142-6. [Crossref] [PubMed]
- Uruga H, Fujii T, Kurosaki A, et al. Pulmonary tumor thrombotic microangiopathy: a clinical analysis of 30 autopsy cases. Intern Med 2013;52:1317-23. [Crossref] [PubMed]
- Miller WT Jr, Shah RM. Isolated diffuse ground-glass opacity in thoracic CT: causes and clinical presentations. AJR Am J Roentgenol 2005;184:613-22. [Crossref] [PubMed]
- Johkoh T, Itoh H, Muller NL, et al. Crazy-paving appearance at thin-section CT: spectrum of disease and pathologic findings. Radiology 1999;211:155-60. [Crossref] [PubMed]
- De Wever W, Meersschaert J, Coolen J, et al. The crazy-paving pattern: a radiological-pathological correlation. Insights Imaging 2011;2:117-32. [Crossref] [PubMed]
- Carmeliet P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005;69 Suppl 3:4-10. [Crossref] [PubMed]
- Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer 2011;2:1097-105. [Crossref] [PubMed]
- Tacke F, Schoffski P, Trautwein C, et al. Tissue factor and thrombomodulin levels are correlated with stage of cirrhosis in patients with liver disease. Blood Coagul Fibrinolysis 2001;12:539-45. [Crossref] [PubMed]
- Calnek DS, Grinnell BW. Thrombomodulin-dependent anticoagulant activity is regulated by vascular endothelial growth factor. Exp Cell Res 1998;238:294-8. [Crossref] [PubMed]
- Camera M, Giesen PL, Fallon J, et al. Cooperation between VEGF and TNF-alpha is necessary for exposure of active tissue factor on the surface of human endothelial cells. Arterioscler Thromb Vasc Biol 1999;19:531-7. [Crossref] [PubMed]
- Kosem M, Tuncer I, Kotan C, et al. Significance of VEGF and microvascular density in gastric carcinoma. Hepatogastroenterology 2009;56:1236-40. [PubMed]
- Ding S, Lin S, Dong X, et al. Potential prognostic value of circulating levels of vascular endothelial growth factor-A in patients with gastric cancer. In Vivo 2005;19:793-5. [PubMed]
- Wartiovaara U, Salven P, Mikkola H, et al. Peripheral blood platelets express VEGF-C and VEGF which are released during platelet activation. Thromb Haemost 1998;80:171-5. [Crossref] [PubMed]
- Mirzapoiazova T, Kolosova I, Usatyuk PV, et al. Diverse effects of vascular endothelial growth factor on human pulmonary endothelial barrier and migration. Am J Physiol Lung Cell Mol Physiol 2006;291:L718-24. [Crossref] [PubMed]
- Das S, Ladell DS, Podgrabinska S, et al. Vascular endothelial growth factor-C induces lymphangitic carcinomatosis, an extremely aggressive form of lung metastases. Cancer Res 2010;70:1814-24. [Crossref] [PubMed]
- Wang TB, Wang J, Wei XQ, et al. Serum vascular endothelial growth factor-C combined with multi-detector CT in the preoperative diagnosis of lymph node metastasis of gastric cancer. Asia Pac J Clin Oncol 2012;8:180-6. [Crossref] [PubMed]
- Bilgiç CI, Tez M. Serum VEGF levels in gastric cancer patients: correlation with clinicopathological parameters. Turk J Med Sci 2015;45:112-7. [Crossref] [PubMed]
- Ilhan N, Ilhan N, Ilhan Y, et al. C-reactive protein, procalcitonin, interleukin-6, vascular endothelial growth factor and oxidative metabolites in diagnosis of infection and staging in patients with gastric cancer. World J Gastroenterol 2004;10:1115-20. [Crossref] [PubMed]
- Macedo F, Ladeira K, Longatto-Filho A, et al. Gastric Cancer and Angiogenesis: Is VEGF a Useful Biomarker to Assess Progression and Remission? J Gastric Cancer 2017;17:1-10. [Crossref] [PubMed]
- Lee NK, Lee JH, Ivan C, et al. MALAT1 promoted invasiveness of gastric adenocarcinoma. BMC Cancer 2017;17:46. [Crossref] [PubMed]


