With the rising identification of incidental pancreatic
cystic lesions, clinicians must be aware of the complexity
in their management. First, one must differentiate between
neoplastic mucinous and nonmucinous cysts which are
managed quite differently. Nonmucinous lesions may be
inf lammatory pseudocysts or neoplastic such as serous
cystadenomas, but if accurately characterized, most do not
require resection or long term follow-up. On the contrary,
mucinous neoplasms (comprised of mucinous cystic
neoplasms (MCN) and intraductal papillary mucinous
neoplasms (IPMN)) have a known premalignant potential,
and therefore are either resected or monitored in a
surveillance program.
The critical issue being faced in routine clinical practice
is accurate preoperative characterization of cystic lesions.
Histology remains the gold standard, but requires resection.
Since that is impractical for most low risk lesions, imaging
provides indirect evidence of morphology. Characterization
of cyst fluid has been touted as a more accurate means define
the nature of pancreatic cysts. Cyst fluid CEA obtained
at time of endoscopic ultrasound fine needle aspiration
(EUS/FNA) remains the most accurate test to distinguish
mucinous from non-mucinous cysts, though its diagnostic
accuracy remains roughly 80% (
1). Unfortunately, the
performance of cytology is poor as well, due in part to
the lack of cellularity in aspirates (
2). The fact that 1 in 5
patients may be incorrectly characterized by state of the art evaluation remains an enormous challenge in daily patient
management leading experts to question the value of the
test for routine cyst characterization.
In 2006, International Consensus Guidelines were
developed by a team of experts to define management of
cystic mucinous neoplasms (
3). They emphasize that the
decision to undergo surgical resection versus surveillance
of a presumed neoplastic cyst should be tempered by the
patient’s wishes, comorbidities, life expectancy and the risk
of malignancy versus the risk of surgery. If the patient is an
appropriate surgical candidate, the guidelines recommend
resection of all MCNs, any IPMN which involve the
main duct or side-branch IPMN (SB-IPMN) which are
symptomatic, have a solid component, or are greater than
3cm in size (
3). Cysts without these worrisome features
should be monitored by imaging at 6-12 month intervals.
While these recommendations appear straightforward,
there remain unresolved challenges in their application
to patient management. According to the guidelines,
one should distinguish between MCN and IPMN, and
in particular focal SB-IPMN, since the former should be
resected whereas the latter can be monitored.
To date, imaging alone or combined with a battery of
tests (fluid analysis, serum markers) fail to adequately
addresses these challenges. Thus guidelines must rely on
a presumptive diagnosis based on imperfect tools, which
as expected, lead to imperfect selection of patients for
surgical intervention. Given the morbidity and mortality of
pancreatic surgery, it is not surprising efforts to better select
patients for resection are a source of active investigation.
Al-Rashdan et al. attempt to critically evaluate this
confusing maze of data and ask whether cyst fluid analysis
really addresses this unmet clinical quandary of how to
appropriately select patients with pancreatic cysts for
surgery (
4). They focus on the challenge to distinguish
between mucinous subtypes by evaluating cyst fluid CEA
and amylase. In the 10 year study period, they identified 134 patients with pancreatic cysts who underwent surgical
resection. Of these patients, 82 underwent a preoperative
EUS. Sixty-six of the 82 were mucinous cysts (14 MCN, 52
IPMN). Of these 66, 25 had preceding FNA and cyst fluid
analysis performed (9 MCN, 11 SB-IPMN and 5 main duct
IPMN). The median and mean CEA were not statistically
different between the 9 MCN and all 16 IPMN (p=0.19),
as well as, MCN and SB-IPMN (p=0.34). The median and
mean amylase were not statistically different between the
MCN and all IPMN (p=0.64) and MCN and SB-IPMN
(p=0.92). Of note, no data was provided regarding crosssectional
imaging or EUS findings.
Their data is similar to other studies that have found
limitations in the accuracy of cyst fluid CEA and amylase--
as well as its selective utilization in practice. In a cohort of
33 mucinous cystadenomas and 235 IPMN patients (
5),
Slozek et al. showed that neither CEA nor amylase was
unable to distinguish between mucinous cystadenomas
and IPMN (p=0.26 and 0.23 respectively). However, for
this study, how many of the pathologic diagnoses were
confirmed by surgical pathology or how the definition
of mucinous cystadenoma was made was not provided.
Curiously, cyst fluid CA19-9 was noted to distinguish
mucinous cystadenomas and IPMN (p=0.003) (
5).
The elevated CA19-9 raises the possibility of a different
biomarker to distinguish between types of mucinous cysts.
Another study of 14 MCN and 52 IPMN cases confirmed
by surgical pathology reported median CEA of 2844 ng/ml
(range 1-14,500) in MCN and 574 ng/ml (0-38,500) in
IPMN (
5). While statistical analysis of this difference was
not reported, the overlap between CEA concentrations is
readily apparent. Most recently, in a study of 126 patients,
Park et al. reported overlapping median values cyst f luid
CEA between MCN and IPMN (428ng/ml [interquartile
range IQR: 44-7870] and 414ng/ml [IQR 102-1223]), again
without statistical analysis (
7). Median values (and IQR)
for cyst f luid amylase overlapped as well for MCN and
IPMN (6800 IU/L [IQR 70-25,295] and 5090 IU/L [IQR
1119-38,290], respectively) (
7).
The data from Al-Rashdan et al. adds to the growing
body of evidence that cyst fluid analysis (CEA and amylase)
alone is disappointing in its ability to distinguish between
the mucinous lesions, MCN and IPMN. However, the
question is we would ever look at cyst fluid analysis alone to
make our clinical decisions? The answer is probably not.
The ability to distinguish clinically between the two
mucinous types requires a broader perspective whereby
imaging and patient factors play a well-documented
role. Crippa et al. highlight the clinical and demographic
differences between 168 patients with MCN and 159
with branch-duct IPMN (
8). Patients with MCN were significantly younger (median 44.5 v. 66 yo, p=0.001) and
almost exclusively women (95% v 57%, p=0.01) (
8). MCN
were most likely to be distal (97% v 25%, p =0.001) and
were more likely to present with abdominal pain (62% v
45%, p=0.004) (
8). IPMNs were also more likely to have a
family history of pancreatic cancer (11% v 3.5%, p=0.01)
and a history of other neoplasms (20 v 9%, p=0.006) (
8).
Moreover, MCN are thought to be separate from the main
pancreatic duct whereas side-branch IPMNs are connected
to the main duct. Of course, distinguishing MCN from
SB-IPMN is not always so straightforward as MCN are
reported to be connected to the main duct in up to 20% of
cases (
9).
At the University of Michigan, as well as other expert
centers, multidisciplinary care involving gastroenterologists,
radiologists, and surgeons and oncologists have become
a valuable addition to the care of patients with pancreatic
cysts. Careful review of the patient’s history in the context
of cross-sectional imaging, surgical risks, and an estimate
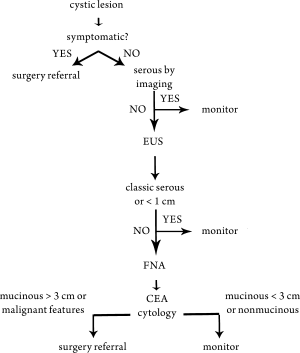
of malignancy risk are taken into account with regard to
clinical decisions. EUS and FNA also play an important
role but are used selectively—it may serve as a confirmatory
role (fluid analysis supporting mucinous etiology or benign
nonmucinous etiology) and for high resolution imaging to
rule out any solid component (See
Fig 1).
What the Al-Rashdan study fails to explore is the clinical
context in which the cyst fluid analysis was drawn. We do
not know demographic information, imaging findings, or
symptoms of the patient. This kind of information is likely
to have played a stronger role than cyst fluid analysis in
distinguishing the two etiologies and in driving the decision
for resection. For example, multifocal cystic disease or an
isolated lesion in the tail in a male is almost certainly IPMN
and may not need resection. The critical question is whether
any type cyst fluid analysis can add incremental value for
such patients—such as prediction of malignancy risk. This
is particularly important in clinically equivocal cases, such
as a woman with a solitary lesion in the body or tail whose
lesion is not clearly distinct from the main duct. In its
current state, CEA and amylase are clearly inadequate and
better biomarkers clearly needed.
There are a number of recent investigations to
evaluate other cyst fluid biomarkers that may aid in the
differentiation of mucinous cyst types. Prostaglandin (
2)
has been shown to have increased expression in pancreatic
cancer tissue over normal pancreatic tissue (
10) and may
also distinguish between types of mucinous cysts. One
study demonstrated that cyst fluid PGE (
2) concentrations
were greater in IPMNs versus MCNs (2.2 ± 0.6 v. 0.2 ±
0.1 pg/mol, p<0.05) (
11). However, there was noted to be an
overlap in PGE (
2) concentrations in benign MCNs and SCAs, thus limiting the utility of this biomarker in the clinical
setting. These findings have not been validated in a larger
study and will require further investigation before it is ready
for clinical application.
Proteomic analysis of cyst fluid in a study of 8 patients
who underwent surgical resection for symptomatic
pancreatic neoplasms identified 92 proteins unique to
MCNs and 29 unique to IPMNs (
12). Analysis identified
several proteins identified in the mucinous lesions (MCN
and IPMN) that were previously reported to be upregulated
pancreatic cancer-associated proteins. The
findings were confirmed by immunohistochemistry for two
of the identified proteins, olfactomedin-4 (OLFM4) and
the cell surface glycoprotein MUC18 (
12). These are very
promising preliminary data which will need to be validated
in future studies.
Using a novel antibody-lectin sandwich array that targets
glycan moieties on proteins (
13), Haab et al. measured
protein expression and glycosylation of MUC1, MUC5AC,
MUC16, CEA, and other proteins associated with
pancreatic cancer in 53 cyst fluid samples (
14). Wheat germ
agglutination of MUC5AC was markedly elevated in MCN
and IPMN but not serous cystadenomas or pseudocysts.
CA19-9 could distinguish between MCN and IPMN with
a sensitivity and specificity of 82% and 93%, respectively.
While these three aforementioned studies of biomarkers are not yet ready for “prime time”, they show potential of
molecular techniques to identify biomarkers that may prove
more useful than CEA or amylase. Much larger sample sizes
will be needed in future validation studies.
This JGO paper reemphasizes that the decision to send
a patient with a pancreatic cyst for resection is complex,
and requires a lot more than just EUS/ FNA with cyst
fluid characterization. Their series confirms the results of
others that amylase levels are of such limited value they
likely should be abandoned. EUS/FNA does have small but
measureable risks of bleeding, infection and pancreatitis;
therefore, we agree with our Indiana University colleagues
and suggest EUS-FNA with CEA levels should be used only
when the results change management. We eagerly await the
identification and development of future biomarkers which
will make “the juice really worth the squeeze.”