Sentinel lymph node in oesophageal cancer—a systematic review and meta-analysis
Introduction
The incidence and mortality from cancer of all types in the United States has decreased during the 1991-2006 timeframe (1). However, the opposite is true for oesophageal cancer. Its incidence and mortality continue to rise. In 2010, estimated new cases of oesophageal cancer number 16,640 in the United States, while deaths total 14,500 (1). The United States has seen an average increase of 20.6% per year in the incidence of adenocarcinoma of the oesophagus since that time (2). This translates into a 463% and 335% increased incidence in white males and females, respectively, between 1975 and 2004. Adenocarcinoma now accounts for 58% of all oesophageal cancers in the United States. Total oesophageal cancer incidence and mortality have been increasing among white men, stable among white women, and decreasing in black men and women (3). It is projected that there will be 16,470 new patients diagnosed with oesophageal cancer and 14,280 deaths from it in 2008 (1).
Oesophageal cancer surgery is one of the most invasive types of gastrointestinal (GI) tract surgery. Due to the recent developments in lymph node metastasis diagnosis, endoscopic mucosal resection and less invasive surgery with thoracoscopic and laparoscopic technique has been possible in early-stage disease. However, because the sites of lymph node metastases are distributed extensively, it is sometimes difficult to focus on the removal of specific lymph nodes, even in superficial oesophageal cancer. The “fear” for the invisible micro metastasis prompted surgeons to perform more aggressive resections with lymphadenectomy to control the disease locally. Given this background, the concept of the sentinel lymph node (SLN), intraoperative lymphatic mapping and sentinel lymphadenectomy appears attractive.
Many studies have validated the sentinel node concept for cutaneous melanoma (4) and breast carcinoma (5,6). The dramatic impact of sentinel node biopsy on clinical practice, most notably for breast carcinoma, has led to recent successful attempts to extrapolate these techniques to other solid tumours including those of the GI tract (7,8). The SLN concept has revolutionized the approach to the surgical staging of both melanoma and breast cancer, and these techniques can benefit patients by avoiding various complications that may result from unnecessary prophylactic radical lymph node dissection in cases of negative SLNs for cancer metastasis. The basic technique used for SLN mapping involves injecting a tracer around the tumour and then following it to where it reaches the first drainage lymph node downstream from the tumour. In other words, the method simply entails the use of tracers and their respective detection devices.
Lymph node metastasis is not a rare event in oesophageal cancer, and the incidence of lymph node metastasis, even in pT1b tumours, reaches 45% (9). The other specific characteristics of oesophageal cancer is multidirectional lymphatic flow from the primary lesion, and the wide spread and random patterns of lymph node metastasis from cervical to abdominal areas. Actually, anatomic skip metastases to the second or third compartment of regional lymph nodes were found in 50% to 60% of oesophageal cancer (9). Based on these clinical observations, extended radical oesophagectomy with 3-field lymph node dissection has become recognized as a standard procedure in Japan, even for clinically node-negative cases (9,10). However, the oesophagectomy with 3-field lymph node dissection is one of the most invasive procedures in GI surgeries. A significant increase of morbidity and mortality after the invasive procedures has been reported (11).
The aim of this meta-analysis was to evaluate the feasibility, reliability, sensitivity and accuracy of sentinel node biopsy for adenocarcinoma, squamous cell carcinoma of the oesophagus.
Methods
Study protocol
We followed the Preferred Reporting Items for Systematic reviews and Meta-Analyses PRISMA guidelines where possible in performing our systematic review (12). We performed a systematic search through MEDLINE (from 1950), PubMed (from 1946), EMBASE (from 1949), Current Contents Connect (from 1998), Cochrane library, Google scholar, Science Direct, and Web of Science to August 2013. The search terms included “Oesophageal cancer” AND “Sentinel Lymph Node Biopsy”, which were searched as text word and as exploded medical subject headings where possible. No language restrictions were used in either the search or study selection. The reference lists of relevant articles were also searched for appropriate studies. A search for unpublished literature was not performed.
Study selection
We included studies that met the following inclusion criteria:
- Studies identifying the population of patients with oesophageal cancer who underwent SLN biopsy;
- Studies that reported sensitivity, negative predictive value and other parameters.
Data extraction
We performed the data extraction using a standardized data extraction form, collecting information on the publication year, study design, number of cases, total sample size, population type, country, continent, mean age and clinical data. The event rate and confidence intervals were calculated.
Statistical analysis
Pooled event rate and 95% confidence intervals were calculated using a random effects model (13). We tested heterogeneity with Cochran’s Q statistic, with P<0.10 indicating heterogeneity, and quantified the degree of heterogeneity using the I2 statistic, which represents the percentage of the total variability across studies which is due to heterogeneity. I2 values of 25%, 50% and 75% corresponded to low, moderate and high degrees of heterogeneity respectively (14). The quantified publication bias using the Egger’s regression model (15), with the effect of bias assessed using the fail-safe number method. The fail-safe number was the number of studies that we would need to have missed for our observed result to be nullified to statistical non-significance at the P<0.05 level. Publication bias is generally regarded as a concern if the fail-safe number is less than 5n+10, with n being the number of studies included in the meta-analysis (16). All analyses were performed with Comprehensive Meta-analysis (version 2.0), Biostat, Englwood, NJ, USA [2005].
Results
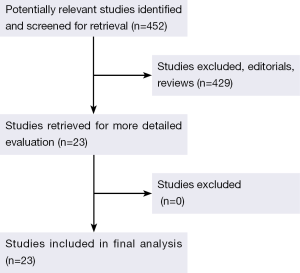
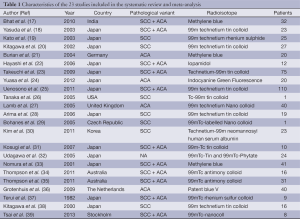
The original search strategy retrieved studies (Figure 1). The abstracts were reviewed and after applying the inclusion and exclusion criteria, articles were selected for full-text evaluation. Of the articles selected, only 22 met full criteria for analysis and are summarised in Table 1. The years of publication ranged from 2002 to 2011.
Full table
Event rates
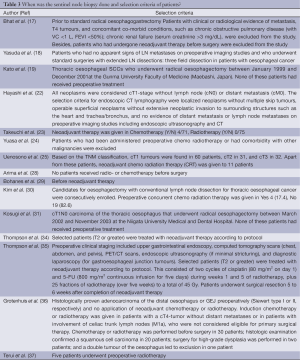
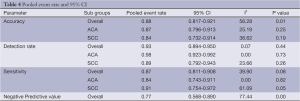
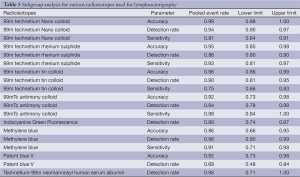
Definitions of various parameters and selection criteria of patients undergoing sentinel node biopsy are listed in Tables 2 and 3 respectively. The overall detection rate was 0.93 (95% CI: 0.894-0.950), sensitivity 0.87 (95% CI: 0.811-0.908), negative predictive value 0.77 (95% CI: 0.568-0.890) and the accuracy was 0.88 (95% CI: 0.817-0.921). In the adenocarcinoma cohort, detection rate was 0.98 (95% CI: 0.923-0.992), sensitivity 0.84 (95% CI: 0.743-0.911) and the accuracy was 0.87 (95% CI: 0.796-0.913). In the squamous cell carcinoma group, detection rate was 0.89 (95% CI: 00.792-0.943), sensitivity 0.91 (95% CI: 0.754-0.972) and the accuracy was 0.84 (95% CI: 0.732-0.914).

Full table
Full table
Heterogeneity and publication bias
The heterogeneity of outcomes has been summarized in Tables 4 and 5. The reason for significant heterogeneity may be attributed to different population groups and the variable type of SLN tracer legislated for clinical use in each country. No publication bias was detected using the Egger’s regression model.
Full table
Full table
Discussion
SLN mapping and biopsy was first applied to melanoma, and was subsequently extended to breast cancer and, more recently, to many other solid tumours including oesophageal cancer (5,6,34,35,42-47). The SLN concept has revolutionized the approach to the surgical staging of both melanoma and breast cancer, and these techniques can benefit patients by avoiding various complications that may result from unnecessary prophylactic radical lymph node dissection in cases of negative SLNs for cancer metastasis. New developments in determining the sentinel node for especially early oesophageal tumours will probably influence operative strategies in the future.
Kuge et al. found direct lymphatic drainage from the submucosal plexus of the oesophagus to the thoracic duct in cadavers. Moreover, they found a long longitudinal extension of the drainage networks of the submucosal plexus suggesting that this is the explanation for skip metastases to cervical nodes (48). Especially early intramural tumours (T1 and T2) probably tend to drain lymph fluid into the longitudinal networks. Therefore, isolated distant lymph node involvement is not necessarily a sign of advanced disease. Regional lymph nodes, on the other hand are connected with tumours invading the extramural layers (T3 and T4) through lymph vessels piercing through the oesophageal wall. In this concept regional lymph nodes would be a sign of more advanced oesophageal cancer. This was confirmed in a retrospective study by Matsubara et al. (49) In order to predict possible sites of sentinel nodes they analysed the location of initial lymph node metastasis in 329 patients after extended oesophagectomy for squamous cell carcinoma. Of the solely positive lymph nodes 82% was located in the relatively distant cervico-thoracic junction or perigastric region. When two or three positive lymph nodes were identified they were rarely confined to the intrathoracic lymph nodes. The authors reasoned that a cervical and abdominal lymph node dissection prior to a thoracotomy can predict intrathoracic node involvement and could be a treatment strategy to prevent a thoracotomy in poor-risk patients (49).
The use of radioactive agents and lymphoscintigraphy to determine the lymphatic spread of oesophageal and gastric cardia cancers is not new (37,50). In 1982 Terui et al. already reported a series of nine patients with oesophageal cancer in whom radioactive sulfur colloid was injected endoscopically around the tumour to visualize mediastinal lymph nodes (37). A total of 106 nodes were removed from the mediastinum and nine of the 12 positive lymph nodes were visualized on the preoperative lymphoscintigram. Of the visualized (hot) nodes, 34.6% was positive while only 3.8% of the nonvisualized (cold) nodes were positive for metastasis. The authors concluded that hot nodes indicate a high percentage probability of metastatic nodes (37). To clarify the lymphatic pathways of the (mainly lower) oesophagus Aikou et al. injected radioactive colloid in the oesophageal submucosa in 19 patients with oesophageal cancer (50). A lymphoscintigraphy was made afterwards. Because they could not find a difference between the radioisotopic uptake by cancer free and metastatic nodes the authors argued that the technique would not have any future role for the diagnosis of lymph node metastases (50). The feasibility of lymphoscintigraphy of the oesophagus was also studied in a canine model (51). After submucosal injection of radiolabeled technetium-99m antimony sulfide colloid in six dogs lymph nodes were identified on nuclear scans. The expected position of lymph nodes based on the scans correlated with the location of the radiolabeled nodes at anatomic dissection (51).
In a study of 16 patients with oesophageal cancer Kitagawa et al. found that the frequency of metastatic involvement in SLNs was significantly higher than in non-sentinel nodes (38). Lymph node involvement was found in only 2% of the non-sentinel nodes. These results were confirmed in a larger study by Yasuda et al. (18). In that study, however, more than 50% of the radioactive nodes were missed by the handheld gamma probe. Lamb et al. who investigated the feasibility and accuracy of the sentinel node concept in 40 patients with oesophageal cancer (27). After routine haematoxylin-eosin and immunohistochemical examination of each lymph node the accuracy was 96% and only two false negative sentinel nodes were identified. Half of the sentinel nodes for lower oesophageal tumours were located in the mediastinum, whereas nearly 75% of the SLNs for gastric cardia cancers were within the abdomen (27). Although less favourable results have been reported as well (32), this study by Lamb et al. has cleared the way for the first clinical applications of the sentinel node concept in oesophageal cancer which hopefully in the future will lead to less extensive lymphadenectomies for patients with negative SLNs.
Instead of lymphoscintigraphy and intra-operative use of a gamma probe after injection of a radioactive colloid other techniques for identifying sentinel nodes have recently been reported (52,53). Near-infrared fluorescent lymph tracers have been tested in a pig model (53). Moreover, the feasibility of an endoscopic computed tomography (CT) lymphography with a new CT contrast agent (iopamidol®) was shown in a canine model and in nine patients with oesophageal squamous cell cancer (52). After contrast injection into the oesophageal submucosa a CT scan was made. With guidance of the CT lymphography all 18 preoperatively identified sentinel nodes in the patients could be resected. Five SLNs in five different patients contained metastases while in those patients no metastases were found in other lymph nodes after formal two- or three-field lymph node dissections. The technique could also visualize lymphatic vessels connecting the tumour sites directly to lymph nodes (52).
Kagoshima University (25) has been the largest cohort published so far and their detection rates of SLNs were 93.3% in cT1, 100% in cT2, 87.5% in cT3, and 45.5% in CRT patients. In the 120 cases where SLNs were identified, lymph node metastases were found in 12 patients with cT1, 18 with cT2, 24 with cT3 tumours, and 3 with CRT. Accuracy rate of SLN mapping was 98.2% in cT1, 80.6% in cT2, 60.7% in cT3, and 40% in CRT patients. Although one false-negative case had cT1 tumour, the lymph node metastasis was detected preoperatively. Multiple studies using a radio-guided approach to find SLNs in oesophageal cancer have reported success rates of 85% to 100%, and accuracy rates of 88% to 96% (19,21,23,27). Grotenhuis et al. (36) recognized a SLN in 98% of patients, nevertheless had an excessively high false negative rate of 15% and an accuracy rate of only 85%. Likewise, Bhat et al. (17) detected a SLN in 81% of patients with an accuracy rate of only 75%. SLN had a sensitivity of 85.71% in mid oesophageal tumours and 93.33% in lower oesophageal tumours. The SLN biopsy had sensitivity of 87.5% in the case of squamous cell carcinoma and 92.86% in the cases of adenocarcinoma of the oesophagus. The accuracy of the procedure for squamous cell carcinoma and adenocarcinoma was 60% and 76.47%, respectively. In our analysis, the overall detection rate was 0.93 (95% CI: 0.894-0.950), sensitivity 0.87 (95% CI: 0.811-0.908), negative predictive value 0.77 (95% CI: 0.568-0.890) and the accuracy was 0.88 (95% CI: 0.817-0.921). In the adenocarcinoma cohort, detection rate was 0.98 (95% CI: 0.923-0.992), sensitivity 0.84 (95% CI: 0.743-0.911) and the accuracy was 0.87 (95% CI: 0.796-0.913). In the squamous cell carcinoma group, detection rate was 0.89 (95% CI: 00.792-0.943), sensitivity 0.91 (95% CI: 0.754-0.972) and the accuracy was 0.84 (95% CI: 0.732-0.914).
Practical problems
Obesity contributes to bigger difficulty in patients with surgical resection and identification of SLNs. The oesophagus is in the posterior mediastinum, it is difficult to recognize lymph node with dye until the mediastinal pleura is opened. Also, in many patients with oesophageal cancer, mediastinal lymph nodes are black due to anthracosis. Therefore, dye method alone may not be suitable for SLN mapping in oesophageal cancer (35). With the use of three serial sections and immunohistochemistry on negative SLNs, 14% (3/22) of patients were upstaged (35) and Lamb et al. also found that 12% (3/25) of pN0 patients were upstaged following immunohistochemistry analysis (27).
The routine use of SLN biopsy in oesophageal cancer cannot alter or limit the extent of lymphadenectomy in the same way as is seen in breast cancer and melanoma. And in oesophageal cancer, preoperative access to sentinel nodes may be as invasive, and as morbid, as the operation itself. But, if one agrees that isolated tumour cells have prognostic significance in oesophageal cancer and are detected in 12-14% of node-negative patients using serial sections and immunohistochemistry, then the SLN concept becomes the only practical method of improving pathological staging (35). So, although sentinel node biopsy has not yet been shown to minimize the extent of lymphadenectomy, it may influence postoperative therapy for a substantial number of patients.
Perhaps the key drawback with SLN biopsy in oesophageal cancer is the variety of SLN tracer authorized for clinical use in each nation (54). The vastly diverse particle sizes hinder wide application of the model and design of a uniform practice. For instance, Japan’s 99mTc-tin colloid (100 nm in size) allows for lymphoscintigraphy 24 h before surgical resection (23), while other smaller radio colloids (like Australia’s 99mTc-antimony trisulphide colloid) have much shorter transit periods in the sentinel nodes (54,55). We observed that the parameters like sensitivity, accuracy and detection rates were higher in radioisotopes when compared to methylene blue or patent blue dyes (Table 6). Facilitating preoperative lymphoscintigraphy in between endoscopic peritumoural injection and same-day surgery is often not practical.
Full table
Skip metastases
Another criticism in the literature about SLN biopsy in oesophageal cancer is the described high frequency of skip metastases, although most of these outcomes have been in patients with squamous cell carcinomas (27,35). If SLNs without metastasis are identified only in abdominal lymph nodes, especially in the tumours limited to the lower third of the oesophagus, cervical lymph nodes dissection may be omitted, such as left thoracotomy or trans-hiatal approach. SLN navigation surgery also is unacceptable for patients who have had neoadjuvant therapy (25).
Limitations
The studies included in the meta-analysis are predominantly from Japan and hence generalization of such results cannot be made. Prospective analysis and long-term follow-up studies of the sentinel node concept are needed before these techniques can be applied widely in different stages, pre or post neoadjuvant chemoradiotherapy, tumour sites and histology. There are considerable variations between particle sizes and particle composition, time between injection and examination. The lack of standardization of technique is a major setback for challenging procedure to gain popularity across the globe. Another important of the studies is lack of uniformity reporting data. Measures are needed to enhance radioisotope develop a swift and precise technique to localise the SLN. In the relative absence of hard facts and in the presence of debatable evidence this procedure cannot be recommended as standard of care at present. More additional, definitive, adequately powered studies with a virtuous selection criteria, predefined surgical technique and that takes into consideration the existing shortcomings of the procedure would be ideal to evaluate the role of SLN biopsy in oesophageal cancers.
Conclusions
SLN biopsy is feasible in oesophageal resections with conservative lymphadenectomy and, when successful, initial results suggest it is very accurate in predicting overall nodal status. However, further work is needed to optimize radiocolloid type, refine the technique and develop a quick and accurate way to determine SLN status intraoperatively. SLN biopsy may become standard of care in oesophageal cancer in the near future, especially in the setting of minimally invasive surgery. Whether it will ever be useful as a tool for tailoring a lymphadenectomy is a question for the future.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Bollschweiler E, Wolfgarten E, Gutschow C, et al. Demographic variations in the rising incidence of esophageal adenocarcinoma in white males. Cancer 2001;92:549-55. [PubMed]
- Ries LAG, Melbert D, Krapcho M, et al. eds. SEER Cancer Statistics Review, 1975-2004, National Cancer Institute, Bethesda, MD 2007.
- Morton DL, Thompson JF, Essner R, et al. Validation of the accuracy of intraoperative lymphatic mapping and sentinel lymphadenectomy for early-stage melanoma: a multicenter trial. Multicenter Selective Lymphadenectomy Trial Group. Ann Surg 1999;230:453-63. [PubMed]
- Giuliano AE, Jones RC, Brennan M, et al. Sentinel lymphadenectomy in breast cancer. J Clin Oncol 1997;15:2345-50. [PubMed]
- Turner RR, Ollila DW, Krasne DL, et al. Histopathologic validation of the sentinel lymph node hypothesis for breast carcinoma. Ann Surg 1997;226:271-6; discussion 276-8. [PubMed]
- Aikou T, Kitagawa Y, Kitajima M, et al. Sentinel lymph node mapping with GI cancer. Cancer Metastasis Rev 2006;25:269-77. [PubMed]
- Kitagawa Y, Ohgami M, Fujii H, et al. Laparoscopic detection of sentinel lymph nodes in gastrointestinal cancer: a novel and minimally invasive approach. Ann Surg Oncol 2001;8:86S-9S. [PubMed]
- Ando N, Ozawa S, Kitagawa Y, et al. Improvement in the results of surgical treatment of advanced squamous esophageal carcinoma during 15 consecutive years. Ann Surg 2000;232:225-32. [PubMed]
- Akiyama H, Tsurumaru M, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg 1994;220:364-72. [PubMed]
- Fujita H, Kakegawa T, Yamana H, et al. Mortality and morbidity rates, postoperative course, quality of life, and prognosis after extended radical lymphadenectomy for esophageal cancer. Comparison of three-field lymphadenectomy with two-field lymphadenectomy. Ann Surg 1995;222:654-62. [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 2009;62:1006-12. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials. 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [PubMed]
- Orwin R. A fail-safe N for effect size in meta-analysis. Journal of educational statistics 1983;1983:157-159.
- Bhat MA, Naikoo ZA, Dass TA, et al. Role of intraoperative sentinel lymph node mapping in the management of carcinoma of the esophagus. Saudi J Gastroenterol 2010;16:168-73. [PubMed]
- Yasuda S, Shimada H, Chino O, et al. Sentinel lymph node detection with Tc-99m tin colloids in patients with esophagogastric cancer. Jpn J Clin Oncol 2003;33:68-72. [PubMed]
- Kato H, Miyazaki T, Nakajima M, et al. Sentinel lymph nodes with technetium-99m colloidal rhenium sulfide in patients with esophageal carcinoma. Cancer 2003;98:932-9. [PubMed]
- Kitagawa Y, Fujii H, Mukai M, et al. Intraoperative lymphatic mapping and sentinel lymph node sampling in esophageal and gastric cancer. Surg Oncol Clin N Am 2002;11:293-304. [PubMed]
- Burian M, Stein HJ, Sendler A, et al. Sentinel node detection in Barrett’s and cardia cancer. Ann Surg Oncol 2004;11:255S-8S. [PubMed]
- Hayashi H, Tangoku A, Suga K, et al. CT lymphography-navigated sentinel lymph node biopsy in patients with superficial esophageal cancer. Surgery 2006;139:224-35. [PubMed]
- Takeuchi H, Fujii H, Ando N, et al. Validation study of radio-guided sentinel lymph node navigation in esophageal cancer. Ann Surg 2009;249:757-63. [PubMed]
- Yuasa Y, Seike J, Yoshida T, et al. Sentinel lymph node biopsy using intraoperative indocyanine green fluorescence imaging navigated with preoperative CT lymphography for superficial esophageal cancer. Ann Surg Oncol 2012;19:486-93. [PubMed]
- Uenosono Y, Arigami T, Yanagita S, et al. Sentinel node navigation surgery is acceptable for clinical T1 and N0 esophageal cancer. Ann Surg Oncol 2011;18:2003-9. [PubMed]
- Tanaka C, Fujii H, Kitagawa Y, et al. Oblique view of preoperative lymphoscintigraphy improves detection of sentinel lymph nodes in esophageal cancer. Ann Nucl Med 2005;19:719-23. [PubMed]
- Lamb PJ, Griffin SM, Burt AD, et al. Sentinel node biopsy to evaluate the metastatic dissemination of oesophageal adenocarcinoma. Br J Surg 2005;92:60-7. [PubMed]
- Arima H, Natsugoe S, Uenosono Y, et al. Area of nodal metastasis and radioisotope uptake in sentinel nodes of upper gastrointestinal cancer. J Surg Res 2006;135:250-4. [PubMed]
- Bohanes T, Neoral C, Aujesky R, et al. Sentinel lymph node in esophageal cancer before neoadjuvant therapy. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2005;149:145-7. [PubMed]
- Kim HK, Kim S, Park JJ, et al. Sentinel node identification using technetium-99m neomannosyl human serum albumin in esophageal cancer. Ann Thorac Surg 2011;91:1517-22. [PubMed]
- Kosugi S, Nakagawa S, Kanda T, et al. Radio-guided sentinel node mapping in patients with superficial esophageal carcinoma: feasibility study. Minim Invasive Ther Allied Technol 2007;16:181-6. [PubMed]
- Udagawa H. Sentinel node concept in esophageal surgery: an elegant strategy. Ann Thorac Cardiovasc Surg 2005;11:1-3. [PubMed]
- Nomura T, Onda M, Miyashita M, et al. Wide-spread distribution of sentinel lymph nodes in esophageal cancer. J Nippon Med Sch 2001;68:393-6. [PubMed]
- Thompson SK, Bartholomeusz D, Devitt PG, et al. Feasibility study of sentinel lymph node biopsy in esophageal cancer with conservative lymphadenectomy. Surg Endosc 2011;25:817-25. [PubMed]
- hompson SK, Bartholomeusz D, Jamieson GG. Sentinel lymph node biopsy in esophageal cancer: should it be standard of care? J Gastrointest Surg 2011;15:1762-8. [PubMed]
- Grotenhuis BA, Wijnhoven BP, van Marion R, et al. The sentinel node concept in adenocarcinomas of the distal esophagus and gastroesophageal junction. J Thorac Cardiovasc Surg 2009;138:608-12. [PubMed]
- Terui S, Kato H, Hirashima T, et al. An evaluation of the mediastinal lymphoscintigram for carcinoma of the esophagus studied with 99mTc rhenium sulfur colloid. Eur J Nucl Med 1982;7:99-101. [PubMed]
- Kitagawa Y, Fujii H, Mukai M, et al. The role of the sentinel lymph node in gastrointestinal cancer. Surg Clin North Am 2000;80:1799-809. [PubMed]
- Tsai JA, Celebioglu F, Lindblad M, et al. Hybrid SPECT/CT imaging of sentinel nodes in esophageal cancer: first results. Acta Radiol 2013. [Epub ahead of print]. [PubMed]
- Grotenhuis BA, van Hagen P, Wijnhoven BP, et al. Delay in diagnostic workup and treatment of esophageal cancer. J Gastrointest Surg 2010;14:476-83. [PubMed]
- Fujii H, Kitagawa Y, Kitajima M, et al. Sentinel nodes of malignancies originating in the alimentary tract. Ann Nucl Med 2004;18:1-12. [PubMed]
- Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg 1992;127:392-9. [PubMed]
- Alex JC, Sasaki CT, Krag DN, et al. Sentinel lymph node radiolocalization in head and neck squamous cell carcinoma. Laryngoscope 2000;110:198-203. [PubMed]
- Ueda K, Suga K, Kaneda Y, et al. Radioisotope lymph node mapping in nonsmall cell lung cancer: can it be applicable for sentinel node biopsy? Ann Thorac Surg 2004;77:426-30. [PubMed]
- Viehl CT, Guller U, Cecini R, et al. Sentinel lymph node procedure leads to upstaging of patients with resectable colon cancer: results of the Swiss prospective, multicenter study sentinel lymph node procedure in colon cancer. Ann Surg Oncol 2012;19:1959-65. [PubMed]
- Orsenigo E, Tomajer V, Di Palo S, et al. Sentinel node mapping during laparoscopic distal gastrectomy for gastric cancer. Surg Endosc 2008;22:118-21. [PubMed]
- McMasters KM. Sentinel-node biopsy in breast cancer. N Engl J Med 2003;349:1968-71; author reply 1968-71.
- Kuge K, Murakami G, Mizobuchi S, et al. Submucosal territory of the direct lymphatic drainage system to the thoracic duct in the human esophagus. J Thorac Cardiovasc Surg 2003;125:1343-9. [PubMed]
- Matsubara T, Ueda M, Kaisaki S, et al. Localization of initial lymph node metastasis from carcinoma of the thoracic esophagus. Cancer 2000;89:1869-73. [PubMed]
- Aikou T, Natugoe S, Tenabe G, et al. Lymph drainage originating from the lower esophagus and gastric cardia as measured by radioisotope uptake in the regional lymph nodes following lymphoscintigraphy. Lymphology 1987;20:145-51. [PubMed]
- Baciewicz FA Jr, McNevin MS, Farris RH, et al. Lymphoscintigraphic technique to image canine esophageal lymph nodes. J Invest Surg 2000;13:265-71. [PubMed]
- Suga K, Shimizu K, Kawakami Y, et al. Lymphatic drainage from esophagogastric tract: feasibility of endoscopic CT lymphography for direct visualization of pathways. Radiology 2005;237:952-60. [PubMed]
- Parungo CP, Ohnishi S, Kim SW, et al. Intraoperative identification of esophageal sentinel lymph nodes with near-infrared fluorescence imaging. J Thorac Cardiovasc Surg 2005;129:844-50. [PubMed]
- Mariani G, Erba P, Manca G, et al. Radioguided sentinel lymph node biopsy in patients with malignant cutaneous melanoma: the nuclear medicine contribution. J Surg Oncol 2004;85:141-51. [PubMed]
- Chakera AH, Hesse B, Burak Z, et al. EANM-EORTC general recommendations for sentinel node diagnostics in melanoma. Eur J Nucl Med Mol Imaging 2009;36:1713-42. [PubMed]