
Prognostic value of multiple cytokine analysis in colorectal cancer: a systematic review
Introduction
Colorectal cancer is the third most common site of malignancy and second leading cause of cancer-related deaths worldwide (1). While treatment strategies have improved markedly over recent decades, there is a recognised need to develop ways in which to better risk-stratify patients so that personalised treatment can be offered. Currently, the most widely-used and powerful predictor is disease stage, which is based on the extent of anatomical spread at presentation and their definitions are set out by the American Joint Committee on Cancer (AJCC) and the Union for International Cancer Control (UICC) (2). According to this system of classification, patients with stage II disease fall into a grey area regarding whether to receive adjuvant chemotherapy (3). With a 5-year recurrence rate of twenty per cent, it is considered overtreatment to offer adjuvant therapy to all patients (4), however there is clearly a group of these node-negative patients who have potential to benefit.
Inflammation is considered a hallmark of cancer (5) and a growing body of evidence also links systemic inflammation to outcome in colorectal cancer, notably in the form of the modified Glasgow prognostic score (GPS)—a composite score of C-reactive protein and albumin—and the neutrophil-lymphocyte ratio (6-8). This reflects the systemic nature of even seemingly localised disease, supported also by the majority of recurrences following surgical resection occurring at distant sites (9-12). It has been suggested that systemic inflammation, mediated by circulating cytokines, may increase the likelihood of metastatic deposits developing and progressing by similar mechanisms to those acting on the primary tumour at the microenvironment (13). Circulating cytokines act through a range of inflammatory pathways and data from clinical studies have shown promising markers, including interleukin-6 (IL-6) (14-18) TNF alpha (TNFα) (17,19,20) and interleukin 1β (IL-1β) (17,21).
While these studies have evaluated individual markers and shown them to weakly predict prognosis, experimental evidence has shown individual cytokines to produce numerous and sometimes opposing interactions in disease progression (22-24). It is possible that characterising inflammatory state by profiling multiple cytokines may be a more robust approach than measuring a single inflammatory marker. Therefore, the aim of this systematic review was to examine the literature evaluating multiple cytokine levels and their prognostic role in colorectal cancer, and to determine whether a composite inflammatory score derived from multiple makers outperformed a single marker-based approach.
Methods
Search strategy
The methodology of this systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines (25,26). Searches of the Medline, Embase and Scopus databases were performed on 30th August 2017 using a search strategy that included the terms ‘colon, rectal, colorectal cancer’, and ‘cytokine, cytokines’ and ‘outcome, prognosis, survival, mortality, death, recurrence’. The results were limited to English language, human studies and articles published since the year 2000; studies prior to this were not included due to outdated clinical practices. The corresponding author was contacted in instances where only the abstract was available and the study was subsequently excluded if the available data was incomplete or could not be provided.
Eligibility criteria
Studies examining the association between baseline, peripherally circulating cytokine levels and prognosis in patients with colorectal adenocarcinoma were eligible. Studies were excluded if two or fewer cytokines were evaluated, or the primary outcome measured was the response to chemotherapy.
Method of study review
Data was collected in duplicate by two independent reviewers and a third reviewer consulted on areas of differing opinion. Quality assessment of eligible studies was performed by assessing the risk of bias in the six domains described by Hayden et al. (27). These were, ‘study participation’, ‘study attrition’, ‘prognostic factor measurement’, ‘outcome measurement’, ‘study confounding’, ‘statistical analysis and reporting’. Two reviewers assessed each study independently and each domain was given a score of 0 for high risk, 1 for moderate risk and 2 for low risk of bias. Instances of differing opinion were resolved through discussion with a third reviewer available to adjudicate if necessary.
Results
Description of studies
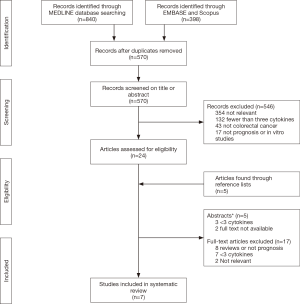
Seven studies were included in this review (17,28-33) after screening 570 records, the number of records screened out by stage are given with the reasons in the flowchart (Figure 1).
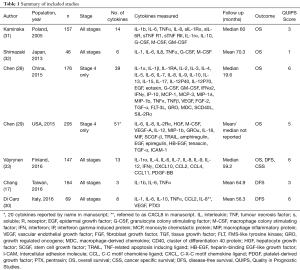
Overall, the quality of the studies was poor to moderate with QUIPS scores ranging from one to six out of twelve (Table 1). Of the six domains assessed, the studies performed well in prognostic factor measurement with a combined score of ten out of fourteen across the seven studies. The authors often reported standardised methods of blood sampling and detailed the assay methods performed. In contrast the studies performed poorly in the ‘study participation’ domain, with a combined score of two. The main reasons for this were small sample sizes, retrospective study design and studies that were limited to the study of stage IV patients.
Full table
Five of the seven studies included patients of all stages (Table 1) (17,30-33). Two of these included all patients attending hospital with a histological new diagnosis of colorectal adenocarcinoma (17,31) and three studies included only those undergoing surgery (30,32,33). The remaining two studies included stage IV patients only (28,29) although a clear rationale for looking exclusively at this sub-group was not given, citing evidence linking circulating cytokines to prognosis in colorectal cancer across patients of all stages (14,35,36).
Two studies were prospective in design (30,33), two retrospectives (17,29) and the study design was not stated in three studies (28,31,32). The sample sizes were relatively modest ranging from 46 to 205 participants and all seven studies were based at separate, single institutions. None of the investigators reported an attempt to perform power calculations to arrive at their intended sample size although the follow up durations across the studies were generally reasonable.
The number and selection of cytokines measured varied across the studies and whilst IL-6 was evaluated in all seven (17,28-33) and IL-8 evaluated in six studies (28-33) based on existing literature, lesser known markers were included in some of the, particularly larger, panels tested (28,29). The Chen et al. [2015 (28) and 2015 (29)] studies utilised commercially available panels including 39 and 51 cytokines, providing justification for only a fraction of the markers tested in the form of existing scientific and clinical evidence. All seven studies measured cytokine levels in pre-treatment samples, with no comparisons to post-operative or follow-up specimens.
The primary end-point measured was overall survival in four studies, and in the remaining three studies, was based on the incidence of disease recurrence (17,30,33). The study by Chang et al. referred to this as the progression-free survival, which was defined as ‘no imaging or pathological evidence of disease progression’ (17). The study included patients of all stages and the treatments undertaken by participants were not stated in the report. Väyrynen et al. measured the disease-free survival (DFS) but did not provide a definition of this outcome in the manuscript (33) and Di Caro et al. measured DFS defined as, ‘any event of local tumour recurrence or any metachronous distant-organ metastases’ (30). For simplicity, the results of these three studies have been grouped together as ‘disease free survival’ in Table 2.
Full table
Di Caro et al. utilised computed tomography, ultrasound and chest radiographs according to a common protocol, and was the only study to report a standard approach to the follow up of their cohort (30).
Individual cytokines and overall survival
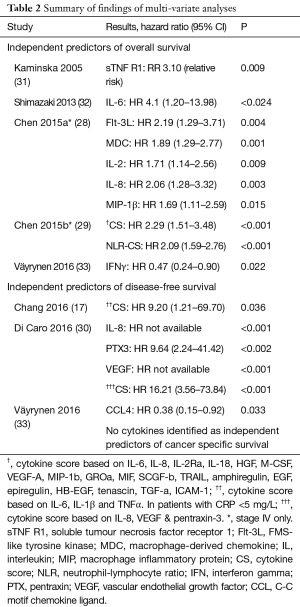
Five studies measured overall survival as the primary outcome (28,29,31-33). On univariate analysis, Chen et al. [2015a] found 17/39 (43.6%), Kaminska 6/14 (42.8%), Shimazaki 1/6 (16.7%) and Väyrynen et al. found none of 13 to be associated with OS whilst Chen et al. [2015b] did not report the results of univariate analysis. Chen et al. [2015a] found 5/39 (12.8%) (28), Chen et al. [2015b] none of 51 (29), Kaminska et al. none of 14 (31), Shimazaki et al. 1/6 (16.7%) (32) and Väyrynen et al. found 1/13 (7.7%) (33) individual cytokines measured to independently predict OS. All five studies included IL-6 in their panel and only one of these identified this cytokine as an independent predictor (32). All five studies included IL-8 and none of them identified it as a predictor. The findings are summarised in Table 2.
One study found that raised levels of interferon gamma (IFNγ) were predictive of a favourable overall survival (33). IFNγ was also included in the panel of one other study—which was limited to stage IV patients—and did not show a significant association with OS on univariate analysis and therefore was not included in multivariate analysis (28). One other marker, CCL4, was identified across the seven studies to predict improved survival with increased levels (33).
Individual cytokines and DFS
Three studies measured DFS. Di Caro et al. found 3/6 (50%) (30), Chang et al. 2/3 (66.6%) (17) and Väyrynen et al. found 1/13 (7.7%) (33) cytokines to be associated with DFS on univariate analysis. On multivariate analysis, Di Caro et al. found 3/8 (37.5%) (30), Chang et al. none of three (17) and Väyrynen et al. found 1/13 (7.7%) (33) individual cytokines to predict DFS. All three studies included IL-6 in their panel but none went on to identify this cytokine as an independent prognostic indicator. IL-8 was found to independently predict DFS in one of the two studies that measured this cytokine (30).
Combined ‘Cytokine Scores’
Four studies combined multiple cytokine levels into a composite score and varied in the number and selection of cytokines as well as the way in which they were combined (17,28-30).
Di Caro et al. incorporated three cytokines that were independent predictors of DFS on multivariate analysis (30). Using cut-off values identified on receiver operator characteristic (ROC) curve analysis, a ‘high’ score was given if all three markers were raised, a ‘low’ score for patients where all three markers were low and a ‘medium’ score for the remaining patients. This score improved upon the prognostic value of pentraxin-3, an acute phase protein related to C-reactive protein (37). The hazard ratios were not available for the remaining two cytokines as all nine events occurred in the patients with raised levels making direct comparison between individual cytokines and the composite cytokine score challenging.
Chang et al. measured three cytokines and incorporated all three into the cytokine score despite only two of which showing a significant association with OS on the Chi squared test (17). Patients were assigned a cytokine score of zero to three based on the number of cytokine values that were above the median. This cytokine score outperformed individual cytokine levels in patients with a CRP of less than 5 mg/L although in patients with CRP above 5 mg/L, individual cytokines and the composite cytokine score were not independent predictors of DFS. The investigators did not justify a rationale for dividing their cohort into high and low CRP groups but did propose cytokine intensity as an alternative indicator of inflammation in colorectal cancer patients (17).
Chen et al. [2015a] included 17 cytokines that were predictive of overall survival, on univariate analysis, into their cytokine score (28). The investigators assigned each cytokine a weighted score, based on their hazard ratio on cox proportional regression. The optimum cut-off value for the cytokine score was then identified from ROC curve analysis to dichotomise the variable into high and low. The investigators did not evaluate their score against important co-variates such as patient and tumour characteristics in a multivariate model but did report a sensitivity and specificity in predicting OS as 0.833 and 0.737, respectively. This out-performed all individual cytokines in its prognostic accuracy.
Chen et al. [2015b] identified 19 cytokines that correlated with neutrophil-lymphocyte ratio and incorporated them into the cytokine score (29). Each patient was given a score based on the sum of the Z scores of the selected cytokines. The Z score was defined as the difference of the average and individual cytokine level divided by standard deviation of log2-transformed value. The cohort was then divided into high or low cytokine score groups using the median score as a cut-off which predicted OS. Individual cytokines were not entered into a cox proportional regression analysis and were therefore not compared directly against the composite cytokine score.
All four studies including a cytokine score concluded that the scores were prognostic (17,28-30). However, only Chen et al. [2015b] made a direct comparison between their composite score and individual cytokines, demonstrating an enhanced prognostic ability (29).
Discussion
This systematic review found that only seven studies evaluated multiple cytokines after 570 records were screened. A high degree of heterogeneity was found between the studies with respect to the patient groups evaluated and outcomes measured. They were also limited by small sample sizes and included those taking an opportunistic look at retrospective cohorts, contributing to poor to moderate quality scores. The use of large, pre-manufactured cytokine panels was sometimes chosen over a targeted investigation of smaller panels of cytokines with known prognostic value and the results of individual cytokine analysis yielded inconsistent findings. In contrast to this, a sub-set of studies combined multiple cytokines to produce a composite cytokine score and these were more consistently found to be predictive, although different methods were used to produce these scores. Furthermore, no reports were found to evaluate different methods of producing a composite score.
The link between inflammation and cancer is considered such that inflammation has been named as one of the hallmarks of cancer (5). This is demonstrated by the up to twenty-fold increase in the lifetime risk of CRC in patients with inflammatory bowel disease, and whilst colitis-associated cancer (CAC) accounts for only 2–3% of CRCs, inflammation also plays an important role in sporadic cancers (38,39). On the cellular level, inflammation within the tumour microenvironment facilitates malignant cell survival, growth and progression by the actions of inflammatory cytokines acting through a variety of pathways including the IL-6/JAK/STAT pathway, which is considered key (6,24). Systemic processes are also thought to play an important role both in recruiting immune cells from the bone marrow and spleen and in facilitating the colonisation of distant organs by metastatic deposits (40). Given the emerging role of systemic inflammation in colorectal cancer, it is clinically relevant to evaluate the prognostic value of circulating inflammatory cytokines.
The ability to predict prognosis is of value to clinicians largely by informing treatment decisions. More aggressive treatment strategies, namely the use of adjuvant chemotherapy following surgical resection may reduce the likelihood of recurrence but results in exposure to potentially harmful side effects and must be administered selectively to those patients considered high-risk. At present, the pathological TNM stage provides the most powerful means of risk-stratifying patients but uncertainty remains, particularly in patients with stage II disease who have a 5-year recurrence rate of approximately 20% (3,4). Two of the included studies were limited to stage IV patients only and a clear rationale for this was not included in the reports (28,29). The results of these studies do not therefore contribute to answer the question of whether multiple cytokines measurement can provide prognostic information that may be used to inform treatment strategy. The two studies limited to stage IV patients also contained variation in the treatment regimes received by participants. In Chen et al.’s [2015b] study (29), 39 patients received fluorouracil, irinotecan and bevacizumab as part of a phase II clinical trial and the treatment undertaken by the remaining 166 patients was not described (41). The patients included in the other study by Chen et al. [2015a] all underwent systemic chemotherapy as their primary treatment and 19 patients underwent partial liver resection, suggesting the cohort included patients undergoing a palliative as well as radical treatment strategy (28). This heterogeneity in study populations diminishes the generalisability of their findings.
The sample sizes were relatively small, ranging from 46 to 205. Furthermore, power calculations were not reported in any of the studies of which only two were prospective in design. The likelihood of type II error may also have been increased by introducing an excessive number of co-variates into the multivariate analyses; a widely accepted rule of thumb being a minimum of ten events being required per variable (42). Recently, calls have been made to relax this rule in certain situations although this is mainly applicable to tests of sensitivity rather than prognostic studies (43). In addition to the number of variables included, the variables themselves are important to consider.
Given that the TNM classification system is universally used to inform treatment strategies, this provides the ideal benchmark to compare cytokine profiles against, yet three studies did not include TNM stage as a co-variate in multi-variate analysis. Cytokine levels have also been shown to vary with stage in clinical studies (44). For these reasons, prognostic studies of cytokine levels should control for disease stage.
Although overall survival is considered the gold standard outcome in prognostic studies, DFS is also an important outcome to measure and is of clinical interest as it reflects disease recurrence. Three of the studies included in this review reported DFS as the primary outcome although it is important to acknowledge that the definition of this outcome varied between them (17,30,33). Additionally, a systematic review by Punt et al. included 52 studies of adjuvant treatment in colon cancer and found wide variation in the definition of the endpoints used and the starting point for measuring time to events (45). This variation in the end-point definitions used must be taken into account when interpreting the DFS results.
All seven of the included studies cited evidence for an overlapping selection of cytokines, frequently including IL-6, IL-8, IL-1β and TNFα, linking systemic inflammation and outcome in colorectal cancer to justify the rationale of their study and yet, in contrast, varied widely in the number and selection of cytokines included in the panels they went on to study (35,36,46). Studies including the lesser known cytokines in their panels were often those implementing multiplex cytokine assays using commercially available, pre-manufactured cytokine panels (28,29). This approach resulted in a generally low-yield in identifying novel biomarkers, one such example of which is Fms-related tyrosine kinase 3 ligand (Flt-3L), shown to independently predict overall survival by Chen et al. [2015a] (28). Flt-3L is a growth and differentiation factor generally associated with haematopoiesis and has been found to predict poor prognosis in acute myeloid leukaemia although its prognostic value in colorectal cancer has not otherwise been reported (47,48).
Two cytokines with a more established prognostic role in colorectal cancer are IL-6 and IL-8 and whilst these cytokines were included in the majority of panels tested, they were only identified as independent predictors in two of the studies (49,50). Given the high level of co-variance between many cytokines, one explanation may be that individual cytokines negate each other in multi-variate analysis. This is especially true of studies including multiple cytokine levels each as co-variates in multivariate analysis.
The four studies that combined multiple cytokine values into a single score all utilised different methods. The question of which cytokines to include is the first consideration and Di Caro et al.’s approach of selecting those cytokines that were predictive of the endpoint independent of stage seems logical (30). In contrast, Chang et al.’s inclusion of a cytokine that had no individual association with outcome would be unlikely to strengthen the predictive power of their combined score (17). The cut-off values used for individual cytokines were either taken as the median, having the advantage of dividing the cohort into equal groups, or were identified through ROC curve analysis. The latter approach is preferable where the data is skewed and this has often been shown to be the case with inflammatory cytokine levels in colorectal cancer populations (44,46). Individual cytokines were weighted according to effect size in the scores used by Chen et al. [2015a] and Chen et al. [2015b] (28,29). This approach acknowledges the finding that some cytokines are more powerful predictors of outcome than others, a theme echoed in the results of each of the studies included in this review as well as many within the wider literature. The finding that two cytokines, IFNγ and CCL4, were found to predict improved rather than worsened survival, in contrast to the remaining prognostic cytokines, only emphasises the need to consider the differential relationships between cytokine value and outcome when formulating a combined score. While the data suggests a multi-marker approach may be useful, no real guidance is provided on which markers to use or how to combine them.
Conclusions
The TNM staging classification, whilst widely used and largely unchanged for many years, fails to predict the significant proportion of patients with node-negative disease who go on to experience poor outcome. Growing evidence supports a link between systemic inflammation and outcome, independent of TNM stage and highlights the importance of the host-response as well as contributing to the reframing of cancer as a systemic disease. As biomarker research continues to identify an increasing number of circulating inflammatory cytokines as potential prognostic predictors, it is clinically pertinent to evaluate the literature assessing multiple cytokine analysis in this context.
This review demonstrates the paucity and heterogeneity of studies examining this topic. There is therefore a need for well-designed prospective studies evaluating a panel of cytokines that is reasonable in number and justified in their inclusion. From this review, prognostic studies evaluating panels of multiple cytokines are relatively ineffective in identifying novel biomarkers and inconsistent in validating more established predictors. Despite these forthcomings, there may be some promise in combining multiple cytokines to produce an enhanced, predictive score.
Acknowledgements
The authors are thankful to the Wellington Surgical Research Trust for their support. The authors would also like to thank the Wellington Medical and Health Sciences Library, especially Jung Cho, for their assistance.
Footnote
Conflicts of Interest: The Wellington Surgical Research Trust has provided support for this research and A Gunawardene is in receipt of a University of Otago Wellington Doctoral Scholarship. The other authors have no conflicts of interest to declare.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4.
- Boland CR, Goel A. Prognostic subgroups among patients with stage II colon cancer. N Engl J Med 2016;374:277-8. [Crossref] [PubMed]
- Marshall JL. Risk assessment in Stage II colorectal cancer. Oncology 2010;24:9. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Guthrie GJK, Roxburgh CSD, Horgan PG, et al. Does interleukin-6 link explain the link between tumour necrosis, local and systemic inflammatory responses and outcome in patients with colorectal cancer? Cancer Treat Rev 2013;39:89-96. [Crossref] [PubMed]
- Oflazoglu U, Alacacioglu A, Kundak Somali I, et al. Prognostic value of neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR) and mean platelet volume (MPV) in patients with colorectal carcinoma Ann Oncol 2016;27:590P. [Izmir Oncology Group (IZOG) study]. [Crossref]
- Li MX, Liu XM, Zhang XF, et al. Prognostic role of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review and meta-analysis. Int J Cancer 2014;134:2403-13. [Crossref] [PubMed]
- Gunawardene A, Desmond B, Shekouh A, et al. Disease recurrence following surgery for colorectal cancer: five-year follow-up. N Z Med J 2018;131:51-8. [PubMed]
- Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer: results of a prospective randomized trial. Arch Surg 1995;130:1062-7. [Crossref] [PubMed]
- Sadahiro S, Suzuki T, Ishikawa K, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 2003;50:1362-6. [PubMed]
- Seo SI, Lim SB, Yoon YS, et al. Comparison of recurrence patterns between≤ 5 years and> 5 years after curative operations in colorectal cancer patients. J Surg Oncol 2013;108:9-13. [Crossref] [PubMed]
- Liu Y, Cao X. Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell 2016;30:668-81. [Crossref] [PubMed]
- Belluco C, Nitti D, Frantz M, et al. Interleukin-6 blood level is associated with circulating carcinoembryonic antigen and prognosis in patients with colorectal cancer. Ann Surg Oncol 2000;7:133-8. [Crossref] [PubMed]
- Bobe G, Albert PS, Sansbury LB, et al. Interleukin-6 as a potential indicator for prevention of high-risk adenoma recurrence by dietary flavonols in the polyp prevention trial. Cancer Prev Res (Phila) 2010;3:764-75. [Crossref] [PubMed]
- Cammarota R, Bertolini V, Pennesi G, et al. Stromal TLR4 and IL 6 expression as potential prognostic inflammation-associated markers for colo-rectal cancer progression. Cancer Res 2010;70:Abstract nr 562.
- Chang PH, Pan YP, Fan CW, et al. Pretreatment serum interleukin-1β, interleukin-6, and tumor necrosis factor-α levels predict the progression of colorectal cancer. Cancer Med 2016;5:426-33. [Crossref] [PubMed]
- Thomsen M, Kersten C, Sorbye H, et al. C-reactive protein and interleukin-6 as markers of systemic inflammatory response and as prognostic factors for metastatic colorectal cancer. Data from the randomized phase III NORDIC-VII study. J Clin Oncol 2015;33:3548.
- Baker EA, El-Gaddal S, Williams L, et al. Profiles of inflammatory cytokines following colorectal surgery: relationship with wound healing and outcome. Wound Repair Regen 2006;14:566-72. [Crossref] [PubMed]
- Chung YC, Chang YF. Serum C-reactive protein correlates with survival in colorectal cancer patients but is not an independent prognostic indicator. Eur J Gastroenterol Hepatol 2003;15:369-73. [Crossref] [PubMed]
- Apte RN, Voronov E. Interleukin-1—a major pleiotropic cytokine in tumor–host interactions. Semin Cancer Biol 2002;12:277-90. [Crossref] [PubMed]
- Lasry A, Zinger A, Ben-Neriah Y. Inflammatory networks underlying colorectal cancer. Nat Immunol 2016;17:230. [Crossref] [PubMed]
- Mager LF, Wasmer MH, Rau TT, et al. Cytokine-Induced Modulation of Colorectal Cancer. Front Oncol 2016;6:96. [Crossref] [PubMed]
- West NR, McCuaig S, Franchini F, et al. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol 2015;15:615-29. [Crossref] [PubMed]
- Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [Crossref] [PubMed]
- Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [Crossref] [PubMed]
- Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280-6. [Crossref] [PubMed]
- Chen ZY, He WZ, Peng LX, et al. A prognostic classifier consisting of 17 circulating cytokines is a novel predictor of overall survival for metastatic colorectal cancer patients. Int J Cancer 2015;136:584-92. [PubMed]
- Chen ZY, Raghav K, Lieu CH, et al. Cytokine profile and prognostic significance of high neutrophil-lymphocyte ratio in colorectal cancer. Br J Cancer 2015;112:1088-97. [Crossref] [PubMed]
- Di Caro G, Carvello M, Pesce S, et al. Circulating inflammatory mediators as potential prognostic markers of human colorectal cancer. PLoS One 2016;11:e0148186. [Crossref] [PubMed]
- Kaminska J, Nowacki M, Kowalska M, et al. Clinical significance of serum cytokine measurements in untreated colorectal cancer patients: soluble tumor necrosis factor receptor type I–an independent prognostic factor. Tumour Biol 2005;26:186-94. [Crossref] [PubMed]
- Shimazaki J, Goto Y, Nishida K, et al. In patients with colorectal cancer, preoperative serum interleukin-6 level and granulocyte/lymphocyte ratio are clinically relevant biomarkers of long-term cancer progression. Oncology 2013;84:356-61. [Crossref] [PubMed]
- Väyrynen JP, Kantola T, Väyrynen SA, et al. The relationships between serum cytokine levels and tumor infiltrating immune cells and their clinical significance in colorectal cancer. Int J Cancer 2016;139:112-21. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [Crossref] [PubMed]
- Ning Y, Manegold PC, Hong YK, et al. Interleukin‐8 is associated with proliferation, migration, angiogenesis and chemosensitivity in vitro and in vivo in colon cancer cell line models. Int J Cancer 2011;128:2038-49. [Crossref] [PubMed]
- Grivennikov SI, Karin M. Inflammatory cytokines in cancer: tumour necrosis factor and interleukin 6 take the stage. Ann Rheum Dis 2011;70:i104-8. [Crossref] [PubMed]
- Garlanda C, Bottazzi B, Bastone A, et al. Pentraxins at the crossroads between innate immunity, inflammation, matrix deposition, and female fertility. Annu Rev Immunol 2005;23:337-66. [Crossref] [PubMed]
- Karin M, Greten FR. NF-κB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol 2005;5:749. [Crossref] [PubMed]
- Grivennikov SI, Cominelli F. Colitis-Associated and Sporadic Colon Cancers: Different Diseases, Different Mutations? Gastroenterology 2016;150:808-10. [Crossref] [PubMed]
- McAllister SS, Weinberg RA. The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol 2014;16:717-27. [Crossref] [PubMed]
- Kopetz S, Hoff PM, Morris JS, et al. Phase II trial of infusional fluorouracil, irinotecan, and bevacizumab for metastatic colorectal cancer: efficacy and circulating angiogenic biomarkers associated with therapeutic resistance. J Clin Oncol 2010;28:453. [Crossref] [PubMed]
- Peduzzi P, Concato J, Feinstein AR, et al. Importance of events per independent variable in proportional hazards regression analysis II. Accuracy and precision of regression estimates. J Clin Epidemiol 1995;48:1503-10. [Crossref] [PubMed]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. Am J Epidemiol 2007;165:710-8. [Crossref] [PubMed]
- Kantola T, Klintrup K, Väyrynen J, et al. Stage-dependent alterations of the serum cytokine pattern in colorectal carcinoma. Br J Cancer 2012;107:1729-36. [Crossref] [PubMed]
- Punt CJ, Buyse M, Kohne CH, et al. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J Natl Cancer Inst 2007;99:998-1003. [Crossref] [PubMed]
- Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients—a summary of published results. Int J Colorectal Dis 2010;25:135-40. [Crossref] [PubMed]
- Rochais C, Cresteil T, Perri V, et al. MR22388, a novel anti-cancer agent with a strong FLT-3 ITD kinase affinity. Cancer Lett 2013;331:92-8. [Crossref] [PubMed]
- Hang H, Yuan S, Yang Q, et al. Multiplex bead array assay of plasma cytokines in type 2 diabetes mellitus with diabetic retinopathy. Mol Vis 2014;20:1137. [PubMed]
- Xia W, Chen W, Zhang Z, et al. Prognostic value, clinicopathologic features and diagnostic accuracy of interleukin-8 in colorectal cancer: A meta-analysis. PLoS One 2015;10:e0123484. [Crossref] [PubMed]
- Wang Z, Wu P, Wu D, et al. Prognostic and clinicopathological significance of serum interleukin-6 expression in colorectal cancer: A systematic review and meta-analysis. Onco Targets Ther 2015;8:3793-801. [Crossref] [PubMed]


