
A Simplified Peritoneal Sarcomatosis Score for patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
Introduction
Soft tissue sarcomas are considered uncommon among adult malignancies since they comprise ~1% of the incidence of adult solid tumors (1). About one third of these sarcomas are reported to originate in the abdominal cavity including the retroperitoneum (2). These solid tumors are notorious for their exceptional tendency to recur locally or distantly even after complete surgical resection (3,4).
Perhaps the most common form of recurrence of intraabdominal sarcomas is peritoneal sarcomatosis (PS) where the tumors disseminate in diffuse seedings and nodules throughout the peritoneal surface (5). Moreover, PS might be present at the time of diagnosis as a primary manifestation of the disease (6).
In either case of PS, primary or recurrent, the prognosis is generally grim with an estimated median OS between 6−14 months (7,8). In addition to their aggressive behavior, sarcomas and PS carry another challenge to the treating physician, which is their poor response to current oncological therapies such as chemotherapy and regional radiation (6).
With the advancement of surgical techniques which address peritoneal surface malignancies (9) and optimization of treatment protocols of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) for peritoneal carcinomatosis (PC), a potential arises for the treatment of PS as well. A consensus was reported in 2008 that CRS/HIPEC in the treatment of PS is an advisable option by the majority of the expert panel if no extraperitoneal disease is evident (10).
Following the maturation milestones of CRS/HIPEC for PC, we learn that patient selection for such an advanced and aggressive intervention is key to achieve the desired improvement in survival, and to define a line where benefit outweighs harm for this complex group of patients based on the extent of disease, performance status, age, and many other factors that can influence the outcome (11-14).
However, studying PS on a similar scale is somewhat difficult given PS has a significantly lower incidence compared to PC. In addition, identifying specific predictors of the surgical outcome is a standing challenge since the majority of the reports stem from small retrospective institutional experiences.
Herein, we aim to analyze our own experience with CRS/HIPEC for PS and use our data to generate a simplified scoring system to stratify the patients based on their disease-related characteristics. This scoring system is an attempt to predict the surgical short- and long-term outcomes, which may, in some instances, guide further treatment based on the specifics of each patient.
Methods
All patients who underwent CRS/HIPEC for PS by a single surgeon (GIS) between 2007−2017 were included. Patient’s data were prospectively maintained in the electronic medical record and retrospectively reviewed under an approved Institutional Review Board (IRB) protocol and used for research purposes with patients’ informed consent (NCT02082886). CRS/HIPEC was considered if the patient has PS limited to the peritoneal cavity without evidence of extraperitoneal dissemination, and whose disease was deemed operable on preoperative imaging. A Simplified Peritoneal Sarcomatosis Score (SPSS) was calculated based on 3 characteristics as follows:
- Symptoms (absent =0, present =1): pain, obstruction, weight loss, and ascites;
- Grade of tumor (low =0, high =1): tumor grade was evaluated according to the three-tiered National Federation of Centers in the Fight against Cancer (FNCLCC) classification. For the purpose of the present study, grade 1 and 2 sarcomas are described as low grade, and grade 3 sarcomas are described as high grade;
- Peritoneal carcinomatosis index (PCI) (PCI ≤10=0, PCI >10=1): was used to score the extent of peritoneal involvement at the time of surgery.
Thus, SPSS ranged between 0−3. Patients were considered to have low SPSS (SPSS-L) if they scored 0−1, and high SPSS (SPSS-H) if they scored 2−3. Cox-regression analysis was applied to confirm the SPSS as an independent predictor of survival. Kaplan-Meier method was used to draw overall survival (OS) and disease-free survival (DFS) plots from the time of CRS/HIPEC. P values <0.05 were considered statistically significant throughout the analysis.
Results
Twenty-five patients were included. Mean age was 51.84±10.75 years, and 13 patients were males (52%). Eleven patients (44%) presented with primary PS, whereas 14 (56%) presented as a recurrence of a previously resected intraabdominal sarcoma. Thirteen patients (52%) were asymptomatic at presentation. The dominant histology was liposarcoma (well differentiated, myxoid, pleomorphic, or dedifferentiated) with 15 patients (60%), 4 patients (16%) were diagnosed with leiomyosarcoma, and 6 (24%) had other histologies. Table 1 summarizes the characteristics of the patients included in the study.
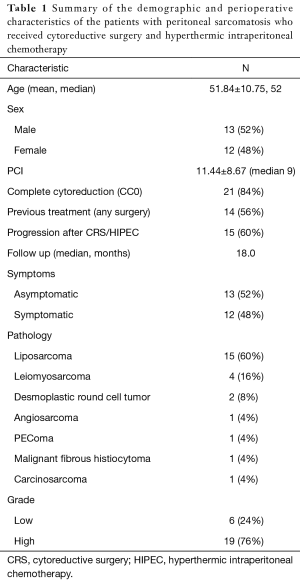
Full table
Mean PCI for the group was 11.44±8.67. Twenty-one patients (84%) achieved complete cytoreduction (CC0), and 4 patients (16%) had CC1-3.
The patients were scored per the description of the SPSS; 4, 9, 3, and 9 patients scored 0, 1, 2, and 3, respectively. Thus, 13 patients were considered SPSS-L and 12 were SPSS-H.
SPSS-L patients were comparable to those with SPSS-H in terms of age, sex, previous treatment (which implies a presentation of primary vs. recurrent disease), and the pathology of their PS. However, it was noted that SPSS-L group had a significantly higher rate of CC0 (100% vs. 66.7%; P=0.027). Table 2 shows the comparison between the two groups.
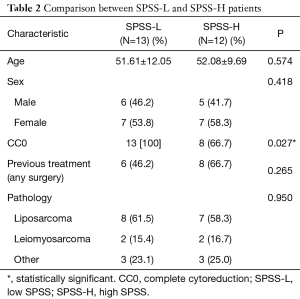
Full table
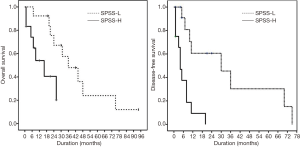
Kaplan-Meier survival analysis demonstrated that SPSS-L had a significantly longer median OS compared to SPSS-H (36±16 vs. 16±6 months; P=0.021), as well as a longer median DFS (30±14 vs. 4±1 months, P<0.001). Figure 1 demonstrates the Kaplan-Meier plots for OS and DFS in both SPSS-L and SPSS-H groups.
To delineate the role of SPSS by adjustment for possible confounders, a cox multivariate regression was applied to OS and DFS. In both models, SPSS was shown to be an independent predictor of OS and DFS. Interestingly, CC0 was a predictor of DFS as expected but was not a predictor of OS. Table 3 summarizes the results of the cox regression for OS and DFS.
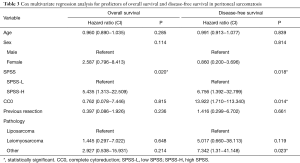
Full table
Discussion
Intraperitoneal sarcomas comprise 18% of all sarcomas (15) and often manifest as PS primarily or after resection as a recurrence. This scenario represents a very challenging theme to the treating oncologist given its obstinance to non-invasive approaches such as radiation and chemotherapy. Currently, the only approach that provided an improved survival for PS is CRS/HIPEC when CC0 is achieved (6,16,17). In the past decade, multiple institutional experiences were reported (16-22) about the impact of CRS/HIPEC on survival in PS and agreed on OS of 24−28 months and DFS of 14−18 months. We have reported that a complete cytoreduction and low PCI score appear to be important factors in the outcome of patient with PS (6). Baratti et al. raised the attention that a variability in the outcomes might be attributed to inherent characteristics at presentation, mostly related to histology, and that patient selection might be advised in this patient population (16). Hence, we aimed to establish a scoring system that would predict the long-term outcomes of CRS/HIPEC in PS based on disease-specific characteristics. In 2009, Pelz and colleagues proved the utility of the peritoneal surface disease severity score (PSDSS) in the prognosis of patients with PC from colorectal origin (23) and was subsequently adopted by the American Society of Peritoneal Surface Malignancies (ASPSM) for stratification of patients with PC from colorectal and ovarian origins (24,25). PSDSS was built on three characteristic pillars; presence of symptoms, radiologic PCI, and histology of the disease, with a significant discrimination in OS between the proposed stages.
In this work, we implement a similar scoring system into PS in a simplified form using our PS population which had comparable OS and DFS to those reported in the literature (26 and 12 months, respectively). Indeed, stratifying patients based on the SPSS into SPSS-L and SPSS-H created an apparent contrast in OS and DFS between the two groups. Since PCI is integrally higher in the SPSS-H group, incomplete cytoreduction would represent a possible confounder that would influence survival. After adjustment for all factors, the multivariate regression analysis proved SPSS to be an independent predictor of OS and DFS. Interestingly, CC0 was only a predictor of DFS but not OS. Given the high tendency of this disease to recur locally, regionally, or remotely, this finding suggest that the improved OS might not be attributed to the complete cytoreduction itself, but rather to the indolent and lenient progression of this disease in this group of patients.
The shortcomings of this study revolve around the small sample size, which cannot be overcome for the rarity of PS cases amenable to CRS and HIPEC. We attempted to limit the bias caused by the small number by confining the stratification to two groups (SPSS-L vs. SPSS-H), and we used a simplified scoring model to avoid the exaggerated complexity that might render this system impractical. Moreover, our PCI score is reported based on the intraoperative evaluation, unlike the PSDSS which was based on preoperative imaging. In our model, this shortcoming can be overcome by considering preoperative radiologic PCI if, based on the institutional volume and experience, correlated adequately with the operative index.
Conclusions
SPSS is a simplified scoring system for PS derived from the PSDSS. It demonstrates an evident survival discrimination between SPSS-L and SPSS-H patients, suggesting that it may be used as tool for patient selection for surgery, prognosis prediction, and stratification into clinical trials in PS.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study is conducted under an approved IRB protocol and patients’ informed consent and the protocol’s name is Edward-Elmhurst Healthcare IRB#1 and its registration number is 0003456.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Jaques DP, Coit DG, Hajdu SI, et al. Management of primary and recurrent soft-tissue sarcoma of the retroperitoneum. Ann Surg 1990;212:51-9. [Crossref] [PubMed]
- Mudan SS, Conlon KC, Woodruff JM, et al. Salvage surgery for patients with recurrent gastrointestinal sarcoma: prognostic factors to guide patient selection. Cancer 2000;88:66-74. [Crossref] [PubMed]
- Rossi CR, Deraco M, De Simone M, et al. Hyperthermic intraperitoneal intraoperative chemotherapy after cytoreductive surgery for the treatment of abdominal sarcomatosis: clinical outcome and prognostic factors in 60 consecutive patients. Cancer 2004;100:1943-50. [Crossref] [PubMed]
- Ng EH, Pollock RE, Munsell MF, et al. Prognostic factors influencing survival in gastrointestinal leiomyosarcomas. Implications for surgical management and staging. Ann Surg 1992;215:68-77. [Crossref] [PubMed]
- Salti GI, Ailabouni L, Undevia S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for the treatment of peritoneal sarcomatosis. Ann Surg Oncol 2012;19:1410-5. [Crossref] [PubMed]
- Bilimoria MM, Holtz DJ, Mirza NQ, et al. Tumor volume as a prognostic factor for sarcomatosis. Cancer 2002;94:2441-6. [Crossref] [PubMed]
- Munene G, Mack LA, Temple WJ. Systematic review on the efficacy of multimodal treatment of sarcomatosis with cytoreduction and intraperitoneal chemotherapy. Ann Surg Oncol 2011;18:207-13. [Crossref] [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Rossi CR, Casali P, Kusamura S, et al. The consensus statement on the locoregional treatment of abdominal sarcomatosis. J Surg Oncol 2008;98:291-4. [Crossref] [PubMed]
- Naffouje SA, Salti GI. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy in Elderly Patients: Complete Cytoreduction Is Feasible and Crucial for Improved Survival Despite High Carcinomatosis Index. Anticancer Res 2018;38:441-8. [PubMed]
- Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of Cytoreductive Surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol 2013;20:3497-503. [Crossref] [PubMed]
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32. [Crossref] [PubMed]
- Kwakman R, Schrama AM, van Olmen JP, et al. Clinicopathological Parameters in Patient Selection for Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Colorectal Cancer Metastases: A Meta-analysis. Ann Surg 2016;263:1102-11. [Crossref] [PubMed]
- Liles JS, Tzeng CW, Short JJ, et al. Retroperitoneal and intra-abdominal sarcoma. Curr Probl Surg 2009;46:445-503. [Crossref] [PubMed]
- Baratti D, Pennacchioli E, Kusamura S, et al. Peritoneal sarcomatosis: is there a subset of patients who may benefit from cytoreductive surgery and hyperthermic intraperitoneal chemotherapy? Ann Surg Oncol 2010;17:3220-8. [Crossref] [PubMed]
- Baumgartner JM, Ahrendt SA, Pingpank JF, et al. Aggressive locoregional management of recurrent peritoneal sarcomatosis. J Surg Oncol 2013;107:329-34. [Crossref] [PubMed]
- Lim SJ, Cormier JN, Feig BW, et al. Toxicity and outcomes associated with surgical cytoreduction and hyperthermic intraperitoneal chemotherapy (HIPEC) for patients with sarcomatosis. Ann Surg Oncol 2007;14:2309-18. [Crossref] [PubMed]
- Sommariva A, Pasquali S, Del Fiore P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients with peritoneal sarcomatosis: long-term outcome from a single institution experience. Anticancer Res 2013;33:3989-94. [PubMed]
- Randle RW, Swett KR, Shen P, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in peritoneal sarcomatosis. Am Surg 2013;79:620-4. [PubMed]
- Bryan ML, Fitzgerald NC, Levine EA, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in sarcomatosis from gastrointestinal stromal tumor. Am Surg 2014;80:890-5. [PubMed]
- Abu-Zaid A, Azzam A, Abuzaid M, et al. Cytoreductive Surgery plus Hyperthermic Intraperitoneal Chemotherapy for Management of Peritoneal Sarcomatosis: A Preliminary Single-Center Experience from Saudi Arabia. Gastroenterol Res Pract 2016;2016:6567473. [Crossref] [PubMed]
- Pelz JO, Stojadinovic A, Nissan A, et al. Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 2009;99:9-15. [Crossref] [PubMed]
- Esquivel J, Lowy AM, Markman M, et al. The American Society of Peritoneal Surface Malignancies (ASPSM) Multiinstitution Evaluation of the Peritoneal Surface Disease Severity Score (PSDSS) in 1,013 Patients with Colorectal Cancer with Peritoneal Carcinomatosis. Ann Surg Oncol 2014;21:4195-201. [Crossref] [PubMed]
- Sleightholm R, Foster JM, Smith L, et al. The American Society of Peritoneal Surface Malignancies Multi-Institution evaluation of 1,051 advanced ovarian cancer patients undergoing cytoreductive surgery and HIPEC: An introduction of the peritoneal surface disease severity score. J Surg Oncol 2016;114:779-84. [Crossref] [PubMed]


