Mucinous adenocarcinoma of the rectum: a poor candidate for neo-adjuvant chemoradiation?
Introduction
Mucinous adenocarcinoma (MA) is diagnosed when more than 50% of the tumor comprises a mucinous pattern upon histological examination (1). MA of the rectum accounts for 5-10% of all adenocarcinomas of the rectum. They have long been recognized to have a poorer survival compared to non-mucinous tumours of the rectum (1). It is believed that mucinous tumours are associated with advanced stage at presentation and the advanced stage rather than the histology is responsible for the worse outcome. The American Joint Committee (2) and the College of American Pathologists (3) consider that MA subtype has not been shown to be statistically significant prognostic factor when matched for similar stage and grade. The guidelines established by the National Comprehensive Cancer Network (NCCN) do not ascribe mucinous histology as a factor that should influence the therapeutic decision making and the current practice is to consider them similar to the non-mucinous tumours and histology does not affect treatment decision making. Consorti et al. (4) have shown that between the two groups, survival was better for nonmucinous than for mucinous tumours. Uni-variate and multi-variate analyses have shown that MA histology is an independent prognostic factor. Mucinous tumours have been shown to have different oncogenic and molecular pathways (5) which may make them respond differently compared to non-mucinous tumours. Tumours with MA are associated with mucus under pressure, which allows the tumour cells to gain access to the peritoneal cavity and have a particular higher chance of occurrence of peritoneal metastasis. Signet ring carcinoma is an epithelial tumour where the predominant component (>50% of the tumour) is made up isolated malignant cells containing intracytoplasmic mucin (1). These tumours have even worse prognosis in the category of mucin secreting tumours. In this study we assess the difference in the pathological response patterns between the mucinous and non-MA of the rectum after a standard course of neoadjuvant chemo-radiation (NACRT).
Material and methods
From 2008-2013, 183 patients who received pre-operative chemo radiotherapy followed by surgery for rectal cancer were evaluated in this study. Patients with biopsy proven rectal cancer were included in this study after a workup consisting of sigmoidoscopy/colonoscopy and a contrast enhanced computed tomography (CT) of the abdomen and pelvis. The tumour was measured on CT scan as determined by size seen in length and maximum thickness of the rectum. The number and size of lymph nodes seen on CT was also noted. The pathological evaluation of the surgical specimens was according to the TNM classification. The patients were divided based on the histology into mucinous (including signet cell variety) and non-MA. As mucin secretion can be induced during NACRT itself, only patients with pre-operative diagnosis of MA were categorized as such in this study. The patients underwent NACRT to a dose of 45 Gy in 25 fractions delivering 1.8 Gy per fraction by four field box technique or 3D conformal radiotherapy (3DCRT). Concurrent weekly 5-flourouracil (5-FU) (350 mg/m2) and leucovorin (20 mg/m2) were administered as a radio sensitizer. The patients underwent abdomino-perineal resection or low anterior resection 4-6 weeks after surgery depending on the distance from the anal verge and depending on the response to NACRT. The descriptive data were analyzed using the Excel 2007 software package.
Results
Patient and tumour characteristics are shown in Table 1. It was seen that patients with MA histology presented at a younger age but showed no significant predilection to gender or smoking habit. The size of initial tumour (as seen on CT scans) and the nodal status were comparable between the two groups.
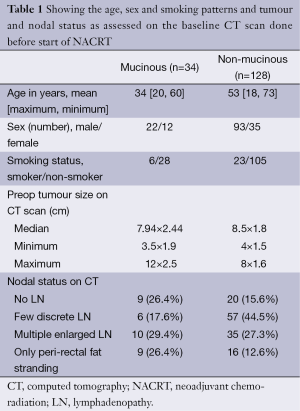
Full table
Six patients with MA of the rectum did not complete the planned treatment as they developed grade 3 enteritis during the course of radiation. Seven patients were lost to follow-up after NACRT and ten patients developed distant metastasis (including peritoneal dissemination) during the course of NACRT and were not offered surgery. These patients were subsequently excluded from the analysis (Table 2). The time delay from NACRT completion to surgery was similar in the two groups.
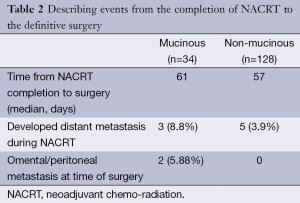
Full table
The pathological response seen in the two groups after NACRT are described in Table 3. The majority of the residual primary tumour in the MA arm was of pT4 stage (73.5%) whereas in the NM group 10.1% of the residual tumours was of T4 group. The incidence of margin positivity was also higher in the MA group (11.7%) compared to 2.3% positive margin in the NM group (P=0.016). Also the residual number of lymph nodes (pN2) was greater in the MA 29.4% vs. 9.3% in the NM group. The most striking difference between the two groups was seen in the occurrence complete pathological response after NACRT which was seen in 15 (11.7%) of the NM groups while none of the patients in the MA group had pathological complete response to NACRT (P=0.03).
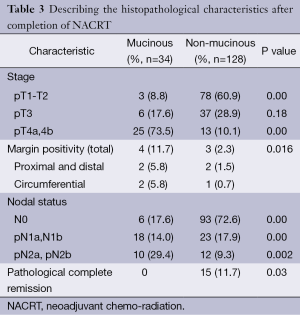
Full table
Discussion
Though mucinous and signet cell varieties have been regarded as a distinct pathological entity, they are treated as for the non-MAs and hence underwent NACRT followed by surgery. Apart from being associated with worse prognosis and poor survival, mucinous histology is an independent prognostic factor and MA have seen to have a different natural history (4-6). Sugarbaker et al. (7) have suggested that MA have mucus under pressure causing the mucus to dissect between the fat planes and carry the tumour cells which float amidst the mucin-“the dissecting mucus theory” which allows the tumour to penetrate deeper and gain access to the peritoneal cavity leading to worse clinical factors including larger primary lesions, deeper invasion, and higher rates of nodal and distant metastasis. Hence peritoneal lavage and intraperitoneal chemotherapy have been proposed during surgery. Molecular and genetic causes may be responsible for the relative radio/chemo resistance of these tumours. The tumour size as evaluated by the CT and the nodal status seen on CT scans were comparable between mucinous and non-mucinous groups. Both the groups underwent NACRT to the same doses and the time interval from completion of NACRT to surgery was also comparable between the two groups. It was seen that patients with MA had poorer response as seen by the post-operative pathological picture of these tumours. Zhang et al. (5) have demonstrated that MA had more K-ras mutations (50% vs. 25%, P=0.02), but less p53 expression (72% vs. 49%, P=0.02) and less apoptotic activity (19% vs. 51%, P=0.01) compared to nonmucinous lesions. In addition, higher rates of loss of heterozygosity and abnormal expression of E-cadherin have been demonstrated in these tumours (6). This observation may be of importance as apoptosis is an important response of the tumour to radiation and has been proposed as predictor of histopathological response to NACRT (8,9). NACRT in rectal cancer is associated with improved local control and survival (10) and the response to NACRT is considered a surrogate marker for oncologic outcome (9,11,12). The presence and number of tumours containing lymph nodes are the most important prognostic factors for survival or recurrence (13). In our study, patients with MA had higher incidence of positive nodes (redundant) after NACRT suggesting poor down-staging and hence are likely to have poor prognosis. Subjecting such poorly responding tumours to NACRT which would delay surgical intervention by 8-10 weeks would also risk tumour dissemination into the peritoneal cavity (7) or development of distant metastasis. Perez et al. (14) studied the PET based response in patients who underwent NACRT and were able to identify a category of poor responders. It was suggested by them that it was not advisable to wait six weeks from the completion of NACRT to the time of surgery. Sengul et al. (15) studied the pathological response after NACRT in different histological types of rectal cancer and demonstrated poorer tumour regression grades after NACRT in the mucinous variety. The limitations of our study is that it is a retrospective study where the preoperative tumour staging was done by CT scan rather than an MRI or transrectal ultrasound which better characterize the depth of the tumour and the lymph nodal stage. However all of the patients did not undergo an MRI preoperatively and hence a CT scan was used to characterize the pre-operative tumour status. Also other pathological features like lympho-vascular invasion, Ki-67 proliferation index, and tumour grade have not been examined in this study for the sake of uniformity as the data was not available in all of the records examined.
Conclusions
MAs of the rectum are a distinct group of tumours which show different natural history, biological behavior and response to NACRT compared to non-mucinous tumours. There may be a lesser value in down staging of tumours which is the principal aim of NACRT. Hence it is recommended that upfront surgery be done in this group rather than the time delay caused due to NACRT which may lead to progression of disease in the form of peritoneal dissemination or development of distant metastasis. Prospective randomized studies are required to verify the true value of NACRT in MA.
Acknowledgements
Mr. Sandeep Kumar and Ms Suman Dhiman, Harvinder Kaur, Savita Bisht for their help in retrieval and organization of records of patients.
Disclosure: The authors declare no conflict of interest.
References
- Symonds DA, Vickery AL. Mucinous carcinoma of the colon and rectum. Cancer 1976;37:1891-900. [PubMed]
- Haggitt RC. International histological classification of tumours: No. 15: Histological typing of intestinal tumours. Dis Colon Rectum 1977;20:697.
- Compton C, Fenoglio-Preiser CM, Pettigrew N, et al. American Joint Committee on Cancer Prognostic Factors Consensus Conference: Colorectal Working Group. Cancer 2000;88:1739-57. [PubMed]
- Consorti F, Lorenzotti A, Midiri G, et al. Prognostic significance of mucinous carcinoma of colon and rectum: a prospective case-control study. J Surg Oncol 2000;73:70-4. [PubMed]
- Zhang H, Evertsson S, Sun X. Clinicopathological and genetic characteristics of mucinous carcinomas in the colorectum. Int J Oncol 1999;14:1057-61. [PubMed]
- Kanazawa T, Watanabe T, Kazama S, et al. Poorly differentiated adenocarcinoma and mucinous carcinoma of the colon and rectum show higher rates of loss of heterozygosity and loss of E-cadherin expression due to methylation of promoter region. Int J Cancer 2002;102:225-9. [PubMed]
- Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990;50:5790-4. [PubMed]
- Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis 1997;12:19-23. [PubMed]
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770-6. [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [PubMed]
- Maas M, Nelemans PJ, Valentini V, et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol 2010;11:835-44. [PubMed]
- Ruo L, Tickoo S, Klimstra DS, et al. Long-term prognostic significance of extent of rectal cancer response to preoperative radiation and chemotherapy. Ann Surg 2002;236:75-81. [PubMed]
- Adell R, Marcote E, Segarra MA, et al. Is mucinous colorectal adenocarcinoma a distinct entity? Gastroenterol Hepatol 2002;25:534-40. [PubMed]
- Perez RO, Habr-Gama A, São Julião GP, et al. Optimal timing for assessment of tumor response to neoadjuvant chemoradiation in patients with rectal cancer: do all patients benefit from waiting longer than 6 weeks? Int J Radiat Oncol Biol Phys 2012;84:1159-65. [PubMed]
- Sengul N, Wexner SD, Woodhouse S, et al. Effects of radiotherapy on different histopathological types of rectal carcinoma. Colorectal Dis 2006;8:283-8. [PubMed]


