How many lymph nodes are enough?—defining the extent of lymph node dissection in stage I–III gastric cancer using the National Cancer Database
Introduction
Gastric cancer affects over 900,000 people worldwide, yet surgical management of the disease varies widely (1). Although the outcome has improved with perioperative chemotherapy or adjuvant chemo-radiation, the primary therapeutic modality for curable gastric cancer remains surgical resection with lymph node dissection (2,3). Nevertheless, the appropriate extent of lymph node dissection is controversial even though the number of positive and negative lymph nodes are independent predictors of disease-free survival (4,5). In Asian countries, extended lymph node dissection (ELND) with D2 or D3 dissection are more favored; while, in western countries, there is less emphasis on ELND (6). Much of the controversy lies in clinical trials demonstrating a benefit to D2 over D1 lymph node dissection in select patients (7,8).
There have been multiple studies addressing the optimal method of gastric lymph node dissection. The Maruyama index, which is a quantitative estimate of residual disease in lymph nodes, has been used to pre-operatively plan such lymph node dissection (9). Moreover, there have been additional studies analyzing large datasets to evaluate a cut-off point on optimal dissection. A Surveillance, Epidemiology, and End Results (SEER) database analysis by Smith et al. demonstrated that 10 lymph nodes dissection was optimal, but there was continued survival improvement with up to 40 lymph nodes removed (10). A study by the U.S. Gastric Cancer Collaborative group reported that dissecting 16 lymph nodes was optimal (11). Even in D2 resection, the optimal lymph node dissection is not known although 16 lymph nodes has been suggested as the minimum number necessary (12).
Herein, the National Cancer Database (NCDB), a comprehensive clinical surveillance resource oncology data set, was used to study the extent of lymph node dissection for resected gastric cancer.
Methods
This analysis used the NCDB from 2004 to 2013 to examine the extent of lymph node dissection in surgically resected gastric cancer with negative margins. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society.
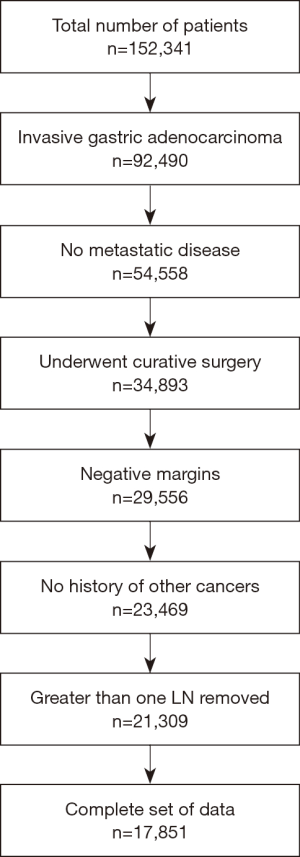
The CONSORT diagram is detailed in Figure 1. Patients with invasive gastric carcinoma treated with surgical resection and lymph node dissection of at least 1 lymph node were included. Exclusion criteria were metastatic disease, positive surgical margin, and incomplete data.
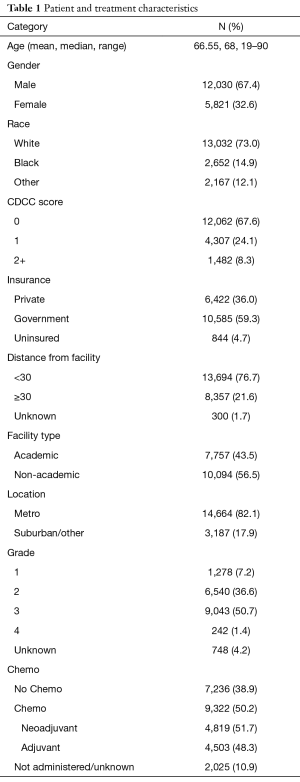
Patient demographics and facility covariates included age, sex, race (whites, blacks, and other), Charlson/Deyo comorbidity score (CDCC) (0 vs. 1 vs. 2+), primary health insurance type (private versus government versus no or unknown insurance), distance in miles from residence to treatment facility (<30 and ≥30), facility type (academic versus non-academic), and location (metro versus urban/other). Clinicopathologic characteristics assessed included year of diagnosis, grade, tumor size, number of positive lymph nodes, and number of lymph nodes removed.
Statistical methods
All statistical analyses were completed by a biostatistician (H Ye). Optimal lymph node cut-off points were determined by Cox regression χ2 score. Each lymph node group cut-offs were tested using Cox regression model, and the one with maximum χ2 was chosen as an optimal cut-off point. Actuarial survival was determined using the Kaplan-Meier method, and comparisons of survival were completed via log-rank tests.
Multiple sensitivity analyses were conducted to limit biases. To prevent stage migration, sensitivity analyses were conducted removing patients who had fewer than 7 LNs dissected. The analysis also removed patients who had fewer than 16 lymph nodes dissected given the AJCC 8th edition defining N3b as metastasis in 16 or more regional LNs. Univariate Cox regression was performed by pathologic nodal stage to examine the relationship between pathologic nodal stage and the lymph node dissection. Receiver operating characteristic (ROC) analysis was completed to determine the optimal cut-off value regarding percent positive lymph nodes.
A multi-variate Cox analysis was conducted using the forward method followed by the backward method. The following variables was included in the regression: age, sex (male vs. female), race (white vs. non-white), CDCC (0 vs. 1 vs. 2+), primary health insurance type (private versus government versus no or unknown insurance), distance in miles from residence to treatment facility (<30 and ≥30), facility type (academic versus non-academic), year of diagnosis, tumor size (<50 vs. ≥50 mm), number of positive lymph nodes (continuous), % positive lymph nodes (<10% vs. ≥10%), number of lymph nodes removed (<20 vs. ≥20), use of chemotherapy, and use of radiation.
A Cox proportional hazard model was then constructed using number of lymph nodes removed as a continuous variable starting with 7 dissected lymph nodes as the reference point to decrease stage migration. Moreover, grade, stage, use of chemotherapy, and use of radiotherapy were corrected in this model.
A two-sided P<0.05 was used to determine statistical significance. SPSS version 24 (IBM, Armonk, NY, USA) was used to perform all statistical analyses. MedCalc Statistical Software version 15.11.3 (MedCalc Software bvba, Ostend, Belgium; https://www.medcalc.org; 2015) and Microsoft Excel 2013 (Microsoft, Redmond, WA, USA) was used for creating graphs.
Results
Patient and treatment characteristics
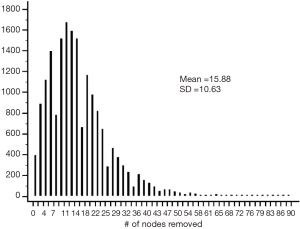
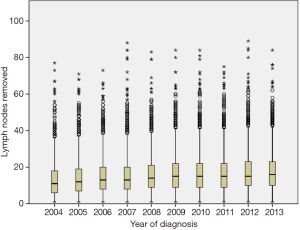
We identified 17,851 patients who fit the inclusion and exclusion criteria. The median follow-up was 37 months (0–130 months). Table 1 shows the patient characteristics. The mean (± SD) number of lymph nodes removed was 16±11 (Figure S1). The number of lymph nodes removed by year is represented in Figure S2, which shows similar median number of lymph nodes removed from 2004 to 2013. The 3- and 5-year survival by stage was 75.8% (95% CI: 74.7–76.9%) and 63.7% (95% CI: 63.7–66.4%) for stage I, 55.5% (95% CI: 54–57.1%) and 43% (95% CI: 41.4–44.8%) for stage II, and 35.4% (95% CI: 34–36.9%) and 25.8% (95% CI: 24.4–27.3%) for stage III, respectively.
Full table
Determining the optimal cut-off point
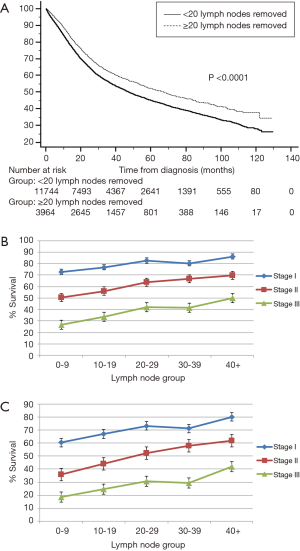
Figure 2 represents the univariate Cox regression χ2 analysis. For the entire cohort, the optimal cut-off point was greater than 20 lymph nodes removed (Figure 2A). When correcting for stage migration (eliminating all patients with less than 7 and 16 lymph nodes removed), the optimal cut-off point continued to be greater than 20 lymph nodes removed (Figure 2B,C). There was a significant overall survival difference [HR 0.80; 95% CI: 0.76–0.84 (P<0.001)] when stratifying patients based on 20 dissected lymph nodes (Figure 3A). When comparing 3- and 5-year survival, the 20–29 lymph node group dissected revealed the most improvement in overall survival (Figure 3B,C).
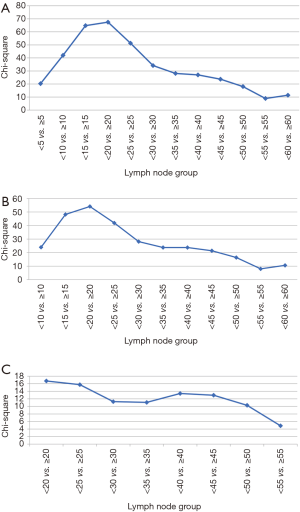
Cox regression and overall survival by pathologic nodal stage
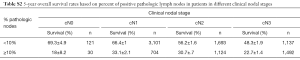
Another sensitivity analysis was conducted looking at number of pathologically positive lymph nodes stratified by pathologic N-stage (Figure S3). The 5-year overall survivals (OS) are shown in Table S1. In patients with 1–2 positive lymph nodes (pN1), dissecting a total of 10 lymph nodes or greater was the cut-off point. For pN1 disease, 3-year OS was 42.8%±1.6% for 0–9 dissected lymph nodes compared to 59.4%±1.1% for 10+ dissected lymph nodes (P<0.001) while the 5-year survival was 30.6%±1.6% for 0–9 dissected lymph nodes compared to 48.2%±1.2% for 10+ dissected lymph nodes (P<0.001). In terms of 3–6 positive lymph nodes (pN2), acquiring a total of 10 or more lymph nodes was also the cut-off point. For pN2 disease, 3-year OS was 28.6%±1.9% for 0–9 dissected lymph nodes compared to 44.1%±1.2% for 10+ dissected lymph nodes (P<0.001) while the 5-year survival was 18.3%±1.7% for 0–9 dissected lymph nodes compared to 32.6%±1.2% for 10+ dissected lymph nodes (P<0.001). Furthermore, for patients with 7+ positive lymph nodes (pN3), dissecting 20 lymph nodes or greater was the cut-point. For pN3 disease, 3-year OS was 25.5%±1.4% for 0–19 dissected lymph nodes compared to 39.3%±1.7% for 20+ dissected lymph nodes (P<0.001) while the 5-year survival was 17.2%±1.3% for 0–19 dissected lymph nodes compared to 28.5%±1.7% for 20+ dissected lymph nodes (P<0.001), respectively.

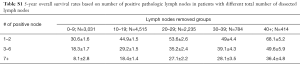
Full table
Cox regression and overall survival by percent positive lymph nodes
A sensitivity analysis was further conducted evaluating the impact of percent positive lymph nodes removed. Percent positive lymph nodes threshold significance was analyzed using ROC analysis (data not shown). The largest change in overall survival was associated with the cut-off point of 10% positive lymph nodes (Table 2). Furthermore, stratification by clinical N stage continued to show the benefit of <10% positive dissected lymph nodes (Table S2).
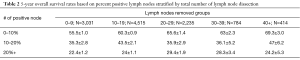
Full table
Full table
Multi-variable Cox regression
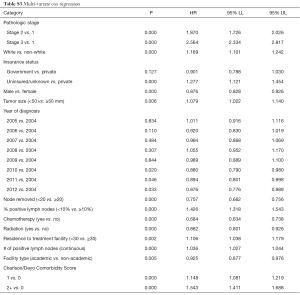
On multi-variable Cox regression analysis (Table S3), higher pathologic stage, non-white race, uninsured/unknown insurance, male sex, tumor size over 50 mm, earlier year of diagnosis, <20 lymph nodes examined, ≥10% positive lymph nodes, lack of chemotherapy, lack of radiation, increased number of positive lymph nodes, non-academic facility, and higher CDCC score were associated with inferior overall survival.
Full table
Cox proportional hazard model
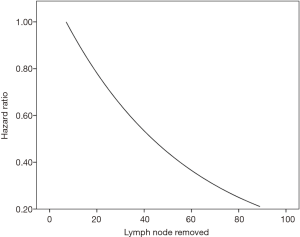
Moreover, a sensitivity analysis was conducted looking at a Cox proportional hazard model using number of lymph nodes removed as a continuous variable while correcting for grade, stage, use of chemotherapy, and use of radiation therapy (Figure S4). There was no optimal cut-off value for lymph node dissection determined via this method as a predictor of overall survival. Hence, it showed that the number of lymph nodes removed could potentially increase the overall survival as a continuous variable.
Discussion
Loco-regional control is paramount in the treatment of gastric cancer. In advanced gastric cancer, treatment usually involves surgical resection ideally with negative surgical margins and adequate lymph node dissection along with neoadjuvant or adjuvant treatment with chemotherapy and/or radiation treatment. The risk of lymph node metastasis in gastric cancer is associated with multiple factors including the histological type, tumor growth pattern, submucosal invasion, and tumor size (13). Lymph node involvement and locoregional spread are common in gastric cancer and overall survival prognosis is highly dependent on the extent of lymph node metastasis. This study solidifies the evidence that a complete dissection is necessary for both staging and therapeutic purposes. Using the NCDB, this analysis indicates that number of lymph nodes dissected is associated with overall survival. Furthermore, dissecting greater than 20 lymph nodes was associated with a superior survival. Greater than 10 lymph nodes removed were necessary with pN1 or pN2 disease while greater than 20 dissected lymph nodes were needed for pN3 disease. Hence, dissecting greater number of lymph nodes in more advanced pathologically staged patients may decrease the risk of “inappropriate understaging” while having a favorable effect on survival. This was also evident as there was better survival with ≤10% positive dissected lymph nodes. However, the question of what is the extent of lymph node dissection to minimize under-staging versus maximizing therapeutic benefit could not be answered in this analysis.
ELND has been adopted in Asian countries, and recent data has begun to change the western countries point of view on ELND in spite of the higher rate of mortality and morbidity associated with D2 dissection per former publications (14). As it was later evident, much of the mortality and morbidity for the D2 dissection is related to the distal pancreatectomy and/or splenectomy (15,16). In 1998, Degiuli et al. published positive results on the feasibility of D2 gastrectomy with pancreas preservation, and the following randomized trial demonstrated no difference in morbidity and mortality between D1 and D2 dissection (8,17,18). The importance of lymph node dissection in overall survival has been the topic of review by many groups. The Dutch Gastric Cancer Group showed no long-term overall survival benefit with D2 lymphadenectomy although this could have been related to increased postoperative mortality (19). Nevertheless, in the subgroup analysis, patients with N2 disease showed improved survival after D2 dissection (19). Subsequently, D2 lymphadenectomy was eventually associated in the long-term with lower loco-regional recurrence and gastric-cancer related deaths (7). Additionally, the Italian Gastric Cancer Study group also reported no significant difference in outcome between D1and D2 dissections (8). However, again subgroup analysis revealed a trend toward benefit with D2 dissection in patients with locally advanced gastric cancer and positive lymph nodes. This concept was further validated when Wu et al. further addressed the extent of lymph node dissection with improved 5-year survival with D3 dissection compared to D1 dissection (20). Furthermore, a meta-analysis by Seevaratnam et al. demonstrated a trend toward survival benefit with D2 dissection for more advanced tumors (T3/T4), and the subgroup analysis of patients with spleen and pancreas preservation continued to show a trend toward better survival rate with D2 compared to D1 (P=0.07) (21). Likewise, meta-analysis by Mocellin et al. reported that D2 dissection was associated with better disease-free survival even though the associated increased rate of post-operative mortality could limit its therapeutic impact (22).
Quantifying the extent of lymph node dissection was further evaluated by Smith et al. using the SEER database, which found a cut-point of 10 lymph nodes yielding the greatest survival difference although there continued to be superior survival up to 40 lymph nodes (10). The U.S. Gastric Cancer Collaborative similarly found that greater than 16 lymph nodes removed had prognostic implications on survival, especially for stage IA–IIIA patients (11). Moreover, a review of the German Gastric Cancer study found that ≤25 lymph nodes and >20% positive lymph node ratio was associated with worse survival in stage II patients (23). Interestingly, the median number of lymph nodes removed in gastric cancer has increased from 7 lymph nodes removed in 2003–2004 as published in the previous NCDB publications to 14 lymph nodes in this study, indicating that clinical practice in the United States favored greater extent of lymph node dissection over the last decade (24). It is possible that ELND is accepted and performed more often in the United States.
As it was discussed above, an interesting finding of this study is that patients with >10% positive dissected lymph nodes have poor outcomes; however, the use of such information clinically can be limited given it would be difficult to obtain such information prior to surgical resection and pathologic review. Moreover, clinical staging in the NCDB has limited utility due to its incomplete information regarding staging work-up and clinical stage determination. Thus, the clinical staging might not be useful in quantifying the minimum number of lymph nodes needed for dissection but could be useful in determining if extended lymph node dissection is needed. It is reasonable to assume the probability of a patient ending with >10% positive dissected LNs is higher with increasing clinical nodal stage. Hence, it is reasonable to consider extended lymph node dissection in such patients, which is in reality can result in therapeutic dissection.
Other limitations of this study are that the NCDB is a large observational database that does not include many clinical and outcomes variables. Hence, there could be an unknown selection bias that is not accounted for as certain variables are not included in the database. Moreover, there is no NCDB coding of D1 versus D2 resection, which could be an important variable in this study and therefore number of lymph nodes dissected was indirectly used instead. Lastly, stage migration is an important concern, although we attempted to correct for this limitation using sensitivity analyses.
In conclusion, the extent of dissected lymph nodes of 20 or greater lymph nodes was associated with superior survival. Extended LN dissection is to be considered especially in patients with clinical lymphadenopathy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by Institutional Review Board (IRB) at Beaumont Health (No. 2016-074). Informed consent is not required as this is a National cancer database study and all data are blinded.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87-108. [Crossref] [PubMed]
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Kodera Y, Schwarz RE, Nakao A. Extended lymph node dissection in gastric carcinoma. J Am Coll Surg 195:855-64. [Crossref] [PubMed]
- Kattan MW, Karpeh MS, Mazumdar M, et al. Postoperative Nomogram for Disease-Specific Survival After an R0 Resection for Gastric Carcinoma. J Clin Oncol 2003;21:3647-50. [Crossref] [PubMed]
- Schmidt B, Yoon SS. D1 Versus D2 Lymphadenectomy for Gastric Cancer. J Surg Oncol 2013;107:259-64. [Crossref] [PubMed]
- Songun I, Putter H, Kranenbarg EM-K, et al. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol 2010;11:439-49. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Randomized clinical trial comparing survival after D1 or D2 gastrectomy for gastric cancer. Br J Surg 2014;101:23-31. [Crossref] [PubMed]
- Kampschöer GHM, Maruyama K, van de Velde CJH, et al. Computer analysis in making preoperative decisions: A rational approach to lymph node dissection in gastric cancer patients. Br J Surg 1989;76:905-8. [Crossref] [PubMed]
- Smith DD, Schwarz RR, Schwarz RE. Impact of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: Data From a Large US-Population Database. J Clin Oncol 2005;23:7114-24. [Crossref] [PubMed]
- Gholami S, Janson L, Worhunsky DJ, et al. Number of Lymph Nodes Removed and Survival after Gastric Cancer Resection: An Analysis from the U.S. Gastric Cancer Collaborative. J Am Coll Surg 2015;221:291-9. [Crossref] [PubMed]
- Xu D, Huang Y, Geng Q, et al. Effect of Lymph Node Number on Survival of Patients with Lymph Node-Negative Gastric Cancer according to the 7th Edition UICC TNM System. PLoS One 2012;7:e38681.
- Roviello F, Rossi S, Marrelli D, et al. Number of lymph node metastases and its prognostic significance in early gastric cancer: A multicenter italian study. J Surg Oncol 2006;94:275-80. [Crossref] [PubMed]
- Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet 1996;347:995-9. [Crossref] [PubMed]
- Degiuli M, Sasako M, Calgaro M, et al. Morbidity and mortality after D1 and D2 gastrectomy for cancer: Interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol 2004;30:303-8. [Crossref] [PubMed]
- Otsuji E, Yamaguchi T, Sawai K, et al. End results of simultaneous pancreatectomy, splenectomy and total gastrectomy for patients with gastric carcinoma. Br J Cancer 1997;75:1219-23. [Crossref] [PubMed]
- Degiuli M, Sasako M, Ponti A, et al. Survival results of a multicentre phase II study to evaluate D2 gastrectomy for gastric cancer. Br J Cancer 2004;90:1727-32. [Crossref] [PubMed]
- Maruyama K, Sasako M, Kinoshita T, et al. Pancreas-preserving total gastrectomy for proximal gastric cancer. World J Surg 1995;19:532-6. [Crossref] [PubMed]
- Bonenkamp JJ, Hermans J, Sasako M, et al. Extended Lymph-Node Dissection for Gastric Cancer. N Engl J Med 1999;340:908-14. [Crossref] [PubMed]
- Wu CW, Hsiung CA, Lo SS, et al. Randomized clinical trial of morbidity after D1 and D3 surgery for gastric cancer. Br J Surg 2004;91:283-7. [Crossref] [PubMed]
- Seevaratnam R, Bocicariu A, Cardoso R, et al. A meta-analysis of D1 versus D2 lymph node dissection. Gastric Cancer 2012;15:S60-9. [Crossref] [PubMed]
- Mocellin S, McCulloch P, Kazi H, et al. Extent of lymph node dissection for adenocarcinoma of the stomach. Cochrane Database Syst Rev 2015.CD001964. [PubMed]
- Siewert JR, Böttcher K, Stein HJ, et al. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg 1998;228:449-61. [Crossref] [PubMed]
- Bilimoria KY, Talamonti MS, Wayne JD, et al. Effect of hospital type and volume on lymph node evaluation for gastric and pancreatic cancer. Arch Surg 2008;143:671-8; discussion 678. [Crossref] [PubMed]