|
Case Report
A 37 year-old pregnant woman with pancreatic adenocarcinoma
treated with surgery and adjuvant chemotherapy:
A case report and literature review
S Lubner1, B Hall2, DV Gopal2, A Soni2, R Hegeman1, N Winterle1, A Loeffler1,3, S Weber1,4, SB Reeder5, N LoConte1
1University of Wisconsin Carbone Cancer Center; 2Department of Medicine, Division of Gastroenterology & Hepatology; Departments of 3Pathology;
4Surgery; 5Radiology, Medical Physics, Biomedical Engineering, and Medicine, University of Wisconsin, Madison, Wisconsin, USA
Corresponding author: Sam Lubner, MD. K4/528 Clinical Sciences Center,
600 Highland Avenue Madison, WI 53792. Email: sjlubner@medicine.wisc.edu
Key words
Pancreatic cancer, pregnancy, gemcitabine, chemotherapy, pancreaticoduodenectomy
J Gastrointest Oncol 2011; 2: 258-261. DOI: 10.3978/j.issn.2078-6891.2011.023
|
|
Case report
A 37 year old G3P1011 pregnant female presented to her
primary care physician with 10 days of nausea, vomiting,
back pain, acholia, and dark colored urine. Her symptoms
worsened as the day progressed. She initially thought the
symptoms were related to her pregnancy, which was 16 weeks
at the time of presentation. She had only minimal symptoms
during the first trimester, and prenatal evaluations/
ultrasounds had all been normal, demonstrating a single
intrauterine pregnancy with appropriate growth for dates.
No familial cancer syndromes were identified, and there were
no known toxic exposures. On initial examination, she was
afebrile, and not in acute distress. Murphy’s sign was present.
No guarding or rebound was demonstrated. She had a serum
bilirubin of 2.8 mg/dL (direct 1.5 mg/dL), and an alkaline
phosphatase of 261 u/L. Hepatitis serologies were negative.
Abdominal ultrasound demonstrated gallstones, no evidence
for cholecystitis, with mild dilation of the intrahepatic and
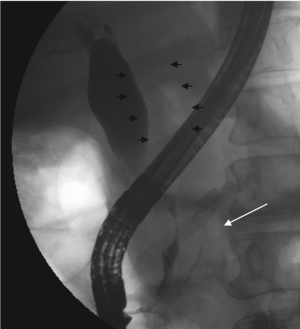
extrahepatic biliary ductal systems. ERCP was performed
the following day which found a distal common bile duct
stricture ( Figure 1). A plastic biliary stent was placed for
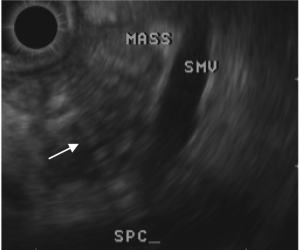
relief of the obstruction. A CA19-9 was elevated at 200 U/mL. Cytology from the ERCP was not revealing, so EUS
(endoscopic ultrasound) with FNA (fine needle aspiration)
was performed two days later ( Figure 2). This returned cells
positive for poorly differentiated adenocarcinoma. Given her pregnancy, consultation with radiology
regarding the most appropriate staging workup was pursued.
CT was inadvisable given the radiation dose, and gadolinium
contrast enhanced MRI was not advised by ACR guidelines
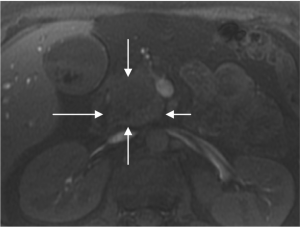
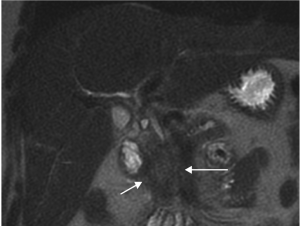
( 1, 2). Non-contrast MRI was performed, which confirmed
the presence of a 2.7 x 3.2 cm mass within the pancreatic
head which abutted, but did not clearly invade the superior
mesenteric vein ( Figure 3&4). Staging laparoscopy with intraoperative ultrasound was
performed. A 2mm lesion was seen and biopsied in segment
2 of the liver, and a single nodule on the surface of the uterus
was biopsied. Both biopsies were negative for malignancy,
and peritoneal washings were negative for malignancy as well.
Fetal heart tones remained normal throughout the case.
With the staging evaluation complete, multidisciplinary
consultation including oncologic surgery, medical
oncology, anesthesiology, and obstetrics was undertaken.
Our institutional preference for neoadjuvant therapy
(chemo+radiotherapy) was not utilized due to the known
teratogenic risk of radiation. After thorough preoperative
discussion of risks and benefits to her and the fetus, she
agreed to undergo pancreaticoduodenectomy. She proceeded
to pancreaticoduodenectomy and cholecystectomy
approximately two weeks after initial presentation. Pathologic
frozen sections of the inferior margin were positive for tumor;
thus, an extended pancreatic resection was performed. A
second frozen specimen was performed of the pancreas
showed no evidence of cancer. Fetal heart tones were normal throughout the case, and the uterus was undisturbed during
the procedure. Postoperative evaluation of fetal heart tones
was normal.
Pathology from the specimen demonstrated poorly
differentiated (grade 3) adenocarcinoma of the pancreas. The tumor was > 5cm in greatest dimension with extension
beyond the pancreas and perineural invasion, but no
involvement of the celiac axis (pT3). Eighteen of 33 lymph
nodes were positive for tumor, and there was extensive invasion of the tumor within associated lymphatic channels
with extranodal extension (pN1). All surgical margins were
negative for carcinoma. The patient recovered well from the
procedure and was discharged to home on postoperative day
six.
Due to the positive margin and tumor stage, adjuvant
gemcitabine was considered ( 3). After a literature review of
available case reports, the risks of teratogenicity and preterm
labor while receiving gemcitabine were approached with the patient and her family. She was willing to proceed. She
received two cycles of gemcitabine (1000 mg/m 2) beginning
her 24 th week of pregnancy, until her 31 st week. She tolerated
chemotherapy well without significant myelosuppression.
Chemotherapy was administered on an inpatient basis to
facilitate fetal monitoring; no adverse fetal effects were seen
during the pregnancy. After a period of washout from her
chemotherapy to minimize the risk of thrombocytopenia in
the infant and mother, labor was induced at 35 weeks and
delivered a male infant (4 pounds 9 ounces) with APGAR
scores of 8 and 9 and blood counts that were within normal
limits. The patient and her baby were monitored in the
hospital and discharged home 6 days after delivery. Given the prolonged period of time off of
chemotherapy, restaging was per formed prior to
reinitiating chemotherapy in an adjuvant strategy. Two
weeks after delivery, and 6 weeks off chemotherapy, CT
scans demonstrated multiple low attenuation lesions
within the liver (largest 1.4 cm), as well as enlarged
mesenteric, aortocaval, and peripancreatic lymph nodes.
With the evidence of recurrence, she was started on a
salvage regimen including capecitabine 1000 mg/m2 po
BID days 1-14, gemcitabine 750 mg/m2 days 4 and 11, and
docetaxel 30 mg/m2 day 1 and 14. She enrolled on a series
of clinical trials and subsequently received many different
chemotherapy regimens but never achieved a durable
response. She died 12 months after diagnosis.
The patient’s child has met all appropriate developmental
milestones in terms of growth, cognitive development,
language development, and socialization. He has a
functionally intact immune system. He is now nearly two
years old.
|
|
Discussion
Other case reports of administration of chemotherapy in
pregnancy have been reported, as have cases of pancreatic
cancer treated surgically in pregnant patients (described
below). We report this case of pancreatic cancer in a pregnant
woman who underwent surgical exploration and adjuvant
chemotherapy, which we believe to be the first case in the
literature.
For her staging, the patient underwent a non-contrast MRI
given theoretical concerns for fetal exposure to gadolinium
based contrast agents. Based on pre-clinical data, animal data,
as well as incidental administration to pregnant patients, the
ACR recommends against the use of gadolinium contrast
agents in pregnancy, and recommends written informed
consent disclosing risks and benefits ( 1). Approaching her surgical procedure, pancreaticoduodenectomy
has been described in the setting of an ampullary tumor in a pregnant woman at 25 weeks’ gestation ( 4), and in pancreatic
adenocarcinoma in at 17 weeks’ gestation ( 5). In another
pregnant patient with pancreatic cancer, labor was induced
at 28 weeks and the patient then proceeded to the operating
room for pancreaticoduodenectomy two weeks later ( 6).
In each of the described cases, no significant adverse fetal
outcomes have been described from the surgical procedures
alone. In all but one of these cases, the maternal outcome was
reported to be uniformly poor. The use of gemcitabine in pregnancy has been described
in non-small cell lung cancer and choriocarcinoma, with
little to no teratogeneic effect when administered after the
first trimester ( 7-9). A single patient received multi-agent
chemotherapy including docetaxel, cisplatin, and gemcitabine
during the first trimester of an unrecognized pregnancy
without significant teratogenesis. Experience in breast cancer,
lymphoma and leukemia suggest that chemotherapy can
be considered in the second and third trimesters after a full
disclosure of the potential risks ( 10, 11). The case described in
this report is the first described in the literature for adjuvant
chemotherapy for pancreatic cancer given while the patient is
still pregnant. No adverse outcome has been seen in the child,
nearly 24 months post delivery. Even with these case reports,
the potential teratogenic effects in the first trimester or during
fetal organogenesis have not been systematically described in
the literature, and this discussion in no way endorses their use
during that phase. This case demonstrates many of the medical and
interpersonal issues that complicate treating pregnant
patients with cancer. In this case, the patient’s primary
goal was to bring a healthy infant to term, understanding
the risks of the proposed treatments to herself and her
fetus during the treatments. With no data to guide in this
specific instance, the treatment team extrapolated data
from other tumor types regarding safety and efficacy of the
chosen treatments. The patient, and all involved physicians
(surgeon, obstetrician, perinatologist, oncologists) were
willing to accept an uncertain degree of risk to help achieve
the patient’s objective of bringing the fetus to term. In
spite of aggressive anticancer therapy, the patient manifest
progressive disease rapidly, and eventually succumbed to
her cancer. There is debate in the oncology community
about the efficacy of neoadjuvant chemotherapy with or
without radiation, and studies are ongoing ( 12, 13). Her
case demonstrates that both locoregional recurrence and
distant recurrence need to be addressed in perioperative
treatment. Her case also highlights the relatively limited
effective treatment options for patients with pancreatic
adenocarcinoma, and underscores the need for research in
the treatment of this disease. |
|
References
- Kanal E, Barkovich AJ, Bell C, Borgstede JP, Bradley WG Jr, Froelich JW,
et al. ACR guidance document for safe MR practices: 2007. AJR Am J
Roentgenol 2007;188:1447-74.[LinkOut]
- Webb JA, Thomsen HS, Morcos SK. The use of iodinated and
gadolinium contrast media during pregnancy and lactation. Eur Radiol
2005;15:1234-40.[LinkOut]
- Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al.
Adjuvant chemotherapy with gemcitabine vs observation in patients
undergoing curative-intent resection of pancreatic cancer: a randomized
controlled trial. JAMA 2007;297:267-77.[LinkOut]
- Ruano R, Hase EA, Bernini C, Steinman DS, Birolini D, Zugaib M.
Pancreaticoduodenectomy as treatment of adenocarcinoma of the papilla
of Vater diagnosed during pregnancy. A case report. J Reprod Med
2001;46:1021-4.[LinkOut]
- Blackbourne LH, Jones RS, Catalano CJ, Iezzoni JC, Bourgeois FJ.
Pancreatic adenocarcinoma in the pregnant patient: case report and review
of the literature. Cancer 1997;79:1776-9.[LinkOut]
- Kakoza RM, Vollmer CM Jr, Stuart KE, Takoudes T, Hanto DW. Pancreatic
adenocarcinoma in the pregnant patient: a case report and literature review.
J Gastrointest Surg 2009;13:535-41.[LinkOut]
- Gurumurthy M, Koh P, Singh R, Bhide A, Satodia P, Hocking M, et al.
Metastatic non-small-cell lung cancer and the use of gemcitabine during
pregnancy. J Perinatol 2009;29:63-5.[LinkOut]
- Kim JH, Kim HS, Sung CW, Kim KJ, Kim CH, Lee KY. Docetaxel,
gemcitabine, and cisplatin administered for non-small cell lung cancer
during the first and second trimester of an unrecognized pregnancy. Lung
Cancer 2008;59:270-3.[LinkOut]
- Pandian Z, Seckl MJ, Smith R, Lees DA. Gestational choriocarcinoma:
an unusual presentation with response to gemcitabine and surgery. BJOG
2004;111:382-4.[LinkOut]
- Cardonick E, Iacobucci A. Use of chemotherapy during human pregnancy.
Lancet Oncol 2004;5:283-91.[LinkOut]
- Ring AE, Smith IE, Jones A, Shannon C, Galani E, Ellis PA. Chemotherapy
for breast cancer during pregnancy: an 18-year experience from five
London teaching hospitals. J Clin Oncol 2005;23:4192-7.[LinkOut]
- Varadhachary GR, Wolff RA, Crane CH, Sun CC, Lee JE, Pisters PW, et
al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based
chemoradiation for resectable adenocarcinoma of the pancreatic head. J
Clin Oncol 2008;26:3487-95.[LinkOut]
- Ota D, Nelson H. Neoadjuvant therapy for potentially resectable pancreatic
adenocarcinoma. Bull Am Coll Surg 2009;94:45-6.
Cite this article as: Lubner S, Hall B, Gopal D, Soni A, Hegeman R, Winterle N, Loeffler A, Weber S, Reeder S, LoConte N. A 37 year-old pregnant woman with pancreatic adenocarcinoma treated with surgery and adjuvant chemotherapy: A case report and literature review. J Gastrointest Oncol. 2011;2(4):258-261. DOI: 10.3978/j.issn.2078-6891.2011.023
|