Breast cancer resistance protein (BCRP) and excision repair cross complement-1 (ERCC1) expression in esophageal cancers and response to cisplatin and irinotecan based chemotherapy
Introduction
Breast cancer resistance protein (BCRP)/ABCG2/MXR/ABCP is a member of the ATP-binding cassette superfamily of transporters present on cell membranes, which was first identified in Adriamycin resistant breast cancer cell lines (MCF-7/AdrVp) (1). It was found to confer resistance to mitoxantrone, doxorubicin, and daunorubicin as it reduced intra-cellular accumulation and retention of these drugs. Apart from tumor cells, it is widely expressed in the body, in stem cells and apical membranes of epithelial cells involved in drug metabolism and distribution in the liver, intestines, kidneys, placenta and the blood-brain barrier (2).
In addition to endogenous substrates such as estrogens, folic acid and protoporphyrin, a number of antitumor drugs (e.g., mitoxantrone, topotecan, methotrexate, irinotecan and flavopiridols) and toxins are substrates of BCRP. Doxorubicin and mitoxantrone interfere with topoisomerase II activity while topotecan inhibits topoisomerase I. All three drugs are substrates of BCRP which would explain cross-resistance to these drugs by reduced cellular drug accumulation due to BCRP expression (3). Irinotecan and its active metabolite SN-38 are camptothecin analogues similar to topotecan. BCRP mediated resistance to camptothecins in human ovarian cancer cells lines (T8 and MX3) was overcome by using an inhibitor of BCRP (GF120918) (4). In another study, co-administration of topotecan with GF120918 in a group of patients was found to increase the bioavailability of toptecan by approximately two fold (5). Other inhibitors of BCRP include imatinib, gefitinib, taxanes, HIV protease inhibitors, glucocorticoids, diethylstilbesterol, tamoxifen, fumitremorgin C and novobiocin (2). Understanding the interaction BCRP substrates and BCRP inhibitors may help us to make rational decisions regarding chemotherapy drug combinations for those cancers which show BCRP expression.
In addition, BCRP expression has been correlated to clinical outcome with chemotherapeutic agents that are known substrates for BCRP. Higher BCRP expression on acute myeloid leukemic blast cells has been associated with a lower likelihood of achieving complete remission as well as shorter duration of remission (6). BCRP negative patients with locally advanced or metastatic non-small cell lung adenocarcinoma treated with platinum based therapy had higher response rates and overall survival (OS) as compared with BCRP positive patients (7). In patients with locally advanced bladder cancer treated with neo adjuvant therapy, BCRP did not show any prognostic impact (8), although p-glycoprotein expression correlated with shorter progression-free survival and high lung resistance related protein/major vault protein expression was associated with a worse response to neo adjuvant chemotherapy. Photofrin, a photosensitizer used in photodynamic therapy, is also a substrate for BCRP and early lung cancer patients with BCRP expression had decreased antitumor responses compared with those who received a photosensitizer which was not a BCRP substrate (9).
Esophageal carcinoma is an aggressive malignancy and is the eighth most common cancer worldwide (10). In the United States, an estimated 17,990 people will be diagnosed with esophageal cancer in 2013 and 15,210 will eventually die of their disease (11). Most patients have non resectable or metastatic disease on diagnosis and there is currently, no standard first line chemotherapy for patients with locally advanced or metastatic disease, although historically platinum and 5-fluorouracil based regimens have been utilized upfront. More recently, irinotecan has been added to the armamentarium of drugs used for this disease, usually in combination with cisplatin. Response rates with this combination have ranged between 30% and 50% (12,13). Despite the addition of newer drugs, no significant strides have been made and five-year survival remains less than 20% (14). Tumor mediated drug resistance may be one of the mechanisms that lead to decreased response to chemotherapy. If therapy could be individualized based on tumor biology, chemotherapy with a higher likelihood of response rates could be selected, thereby prolonging survival.
Irinotecan is one of the substrates for BCRP. In the current study, we explored if BCRP expression is present in esophageal cancers and if this expression correlates with worse prognosis in patients treated with irinotecan chemotherapy. Based on evidence from earlier studies that excision repair cross complement-1 (ERCC1) overexpression can be associated with poor response to cisplatin in non-small cell lung adenocarcinoma, urothelial carcinoma, gastric carcinoma, head and neck squamous cell carcinoma and biliary tract adenocarcinoma (15-19), we further explored ERCC1 expression in esophageal cancers and correlated survival with cisplatin based chemotherapy.
Methods
Institution Review Board approval was obtained to examine medical records and specimens of patients with locally advanced esophageal carcinoma that had been treated at our institution between January 2000 and November 2007. The primary objective was to examine expression of BCRP in esophageal carcinoma. The secondary objectives included correlation of BCRP expression to survival in those patients treated with irinotecan based chemotherapy and examination of expression of ERCC1 and correlation with survival in patients treated with platinum based therapy.
Paraffin-embedded esophageal cancer specimens were evaluated for BCRP expression by immunohistochemistry using specific anti-BCRP monoclonal antibody BXP-21 (Kamiya Biomed Corp). Sections (5-10 μm) were de-paraffinized, rehydrated, and incubated in 3% H2O2 (15 min) to block endogenous peroxidase activity. Antigen retrieval was performed using citrate buffer and non-specific staining was blocked with 0.03% casein. Primary anti-BRCP antibody was applied at a 1:30 dilution in PBS/Tween (PBST) buffer. Following washes in PBST, sections were incubated in biotinylated goat anti-mouse antibody (Jackson ImmunoResearch Laboratories) at a 1:200 dilution, followed by streptavidin horseradish peroxidase conjugate (Zymed Laboratories, Inc, San Francisco, USA) at a 1:20 dilution and DAKO DAB chromogen solution (K3466). Counterstaining was performed with hematoxylin. Controls for staining specificity involved omission of primary antibody or replacement of the primary antibody with normal goat serum. ERCC1 staining was done using Paraffin sections cut at 4 µm, placed on charged slides, and dried at 60 °C for one hour. Slides were cooled to room temperature, de-paraffinized in three changes of xylene, and rehydrated using graded alcohols. For antigen retrieval, slides were heated in the microwave for 20 minutes in TRS buffer (Dako catalog # S1699), followed by a 15-minute cool down. Endogenous peroxidase was quenched with aqueous 3% H2O2 for 10 minutes and washed with PBS/T. Slides were loaded on a DAKO auto stainer and serum free protein block (Dako catalog # X0909) was applied for 5 minutes, blown off, and ERCC1 antibody was applied for one hour. Labeled polymer HRP anti-Mouse Envision reagent (Dako, catalog # K4007) was applied for 30 minutes, followed by the DAB chromogen (Dako) for 10 minutes. Slides were then counterstained with Hematoxylin, dehydrated, cleared and cover slipped.
Scoring was performed by the pathologist who was blinded to the clinical data. For BCRP staining, BCRP score, membrane or cytoplasm of greater than or equal to 30 was considered positive (calculated by multiplying BCRP intensity and % staining). For ERCC1 staining, a proportion score and H score was determined. Proportion score was 0 if 0% staining, 0.1 if 1% to 9%, 0.5 if 10% to 49%, and 1.0 if 50% or more. This proportion score was multiplied by the staining intensity of nuclei to obtain a final semi quantitative H score. Tumors with an H score exceeding 1.0 (i.e., tumors with a staining intensity score of 2 and with 50% or more positive nuclei or with a staining intensity score of 3 and 10% or more positive nuclei) were deemed to be ERCC1-positive (15).
Fisher’s exact test was used to determine association between BCRP and ERCC1 expression and gender, type of chemotherapy, and histology. Cox proportional hazards model was used for association of BCRP or ERCC1 and OS. To test the association between ERCC1 and stage, the Wilcoxon test was used. Categorical variables were summarized using frequencies and relative frequencies. A 0.05 nominal significance level was used in all testing.
Results
Demographic distribution
Forty patients were included in the study of which 35 were male and 5 were female. Median age at diagnosis was 66.5 years with a range of 40 to 90 years. Thirty-eight patients belonged to white ethnicity and one patient each was of African American and native Indian background.
Clinical & treatment characteristics and histopathology
The clinical characteristics of the patients included in the study, are described in Table 1. Most patients had advanced stage at diagnosis, 2 patients had stage I, 7 patients had stage II, 17 patients had stage III and 10 patients had stage IV disease. In 4 patients, stage was not known. There were 4 squamous cell carcinomas and 36 adenocarcinomas. Of these, 4 originated from the middle third of the esophagus and 36 from the lower third part of the esophagus. Median OS was 19 months. Sixteen patients were treated with cisplatin and irinotecan, eight with oxaliplatin and fluoropyrimidine and sixteen received other first line chemotherapy regimens.
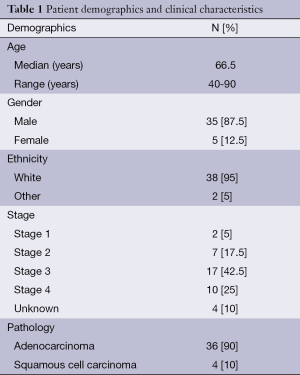
Full table
Breast cancer resistance protein (BCRP)
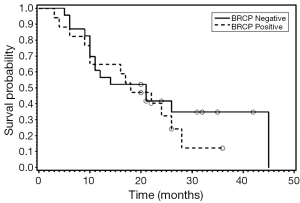
On immunohistochemistry 30 of 40 cancers (75%) expressed BCRP. The distribution was membranous in 17; cytoplasmic in 27 and 14 patients had both cytoplasmic and membranous distribution. Down-regulation of BCRP expression in tumor compared to normal cells was seen in 40% of patients. Median OS was 19 months with no difference in survival between BCRP positive and negative patients (P=0.13). Estimated hazard ratio (HR) of death for BRCP positive patients was 2.29 (95% CI: 0.79-6.64).There was no association between BCRP expression, stage, age, gender or histology. For patients who received cisplatin and irinotecan as first line chemotherapy there was no difference in OS (P=0.39) of BCRP negative versus positive patients.
Excision repair cross complement-1 (ERCC1)
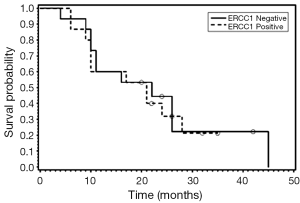
Thirty patients had sufficient sample for ERCC1 staining. Of these, fifteen (50%) were positive for ERCC1. There was no association between ERCC1 expression and gender or histology. There was no significant difference in survival distributions between ERCC1 positive and negative patients (P=0.85). The estimated hazard of death for ERCC1 positive patients is 1.09 (95% CI: 0.46-2.56) times that for ERCC1 negative. For patients who received cisplatin and irinotecan as first line chemotherapy there was no difference in OS (P=0.6299) of ERCC1 positive versus negative patients.
BCRP and ERCC1 expression and co-relation with survival
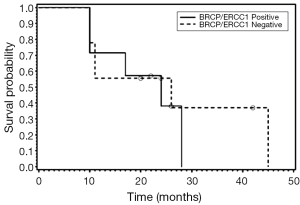
We also examined the association between BRCP and ERCC1 co-expression and OS (Figures 1-3). Seven patients were positive for both BCRP and ERCC1, nine were negative for both, six had positive BCRP and negative ERCC1 expression and eight had negative BCRP and positive ERCC1 expression. There was no association between combined BCRP and ERCC1 overexpression and gender, stage, histology, type of chemotherapy given and OS.
Conclusions
BCRP expression is seen in a majority of esophageal cancers and normal esophageal mucosa. ERCC1 expression is also seen in at least half of esophageal cancers. Response rates to most chemotherapy regimens used in frontline therapy ranges 30% to 50% (20-22). The factors for non-responsiveness to chemotherapy remain to be ascertained. Ours was an exploratory analysis which was hypothesis driven with the intention to translate the results into an effective algorithm for treatment strategy. As we move towards the era of individualized medicine, it would be useful to know upfront, the effectiveness of the proposed chemotherapy in a particular patient. This is crucial in patients with esophageal cancer where the need for predictive markers for adjuvant and neo adjuvant chemotherapy is most felt. In this disease where there is high surgical morbidity and mortality with limited success, there is a demand not only for better chemotherapy drugs but also markers to help predict outcome and better utilization of limited resources.
While BCRP and ERCC1 overexpression in other cancers has been shown to be associated with decreased response to chemotherapy, we could not prove the same in our subset of patients. One of the strengths of our study was that it utilized a validated and reproducible method for examination of BCRP and ERCC1 expression. As we select biomarkers for application into clinical practice, we need to ensure that the methods are standard and easily reproducible. Our study fulfilled these criteria; however, due to our limited sample size we were unable to refute our hypothesis. Furthermore, there may be other genetic and clinical factors that we did not account for which may have affected the results of our study.
Several questions have emerged from this study. To our knowledge, we are the first to demonstrate expression of BCRP in esophageal cancer patients and evidence for down-regulation of BCRP expression in 40% of patients with esophageal cancer as compared with their normal esophageal tissue. It remains to be determined if this down-regulation positively impacts response, and whether the patients that respond to irinotecan based chemotherapy are the ones who show this down-regulation. Most of our specimens had received neoadjuvant therapy and we do not know the effect of chemotherapy on either BCRP or ERCC1 expression; whether there is up or down-regulation on exposure to chemotherapy remains to be determined. With the increasing use of cisplatin and other BCRP substrates such as irinotecan for esophageal cancers, these questions merit further evaluation of BCRP and ERCC1 expression in a larger subset of patients as part of a prospective trial for correlation with response to chemotherapies that may be substrates or modulators of BCRP and ERCC1. Furthermore, BCRP expression can be decreased by epidermal growth factor receptor (EGFR) inhibitors, raising the question if treatment with an EGFR inhibitor could improve clinical outcomes by sensitizing BCRP-positive cancers to therapeutic agents that are BCRP substrates. Successful correlation would allow rational selection of chemotherapies and photosensitizers and individualization of treatments for patients, and would help us to tailor regimens for best clinical outcome.
Acknowledgements
Ms. Donna Oleszek provided technical assistance for the BCRP staining.
Disclosure: The authors declare no conflict of interest.
References
- Doyle LA, Yang W, Abruzzo LV, et al. A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 1998;95:15665-70. [PubMed]
- Staud F, Pavek P. Breast cancer resistance protein (BCRP/ABCG2). Int J Biochem Cell Biol 2005;37:720-5. [PubMed]
- Allen JD, Brinkhuis RF, Wijnholds J, et al. The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 1999;59:4237-41. [PubMed]
- Maliepaard M, van Gastelen MA, Tohgo A, et al. Circumvention of breast cancer resistance protein (BCRP)-mediated resistance to camptothecins in vitro using non-substrate drugs or the BCRP inhibitor GF120918. Clin Cancer Res 2001;7:935-41. [PubMed]
- Kruijtzer CM, Beijnen JH, Rosing H, et al. Increased oral bioavailability of topotecan in combination with the breast cancer resistance protein and P-glycoprotein inhibitor GF120918. J Clin Oncol 2002;20:2943-50. [PubMed]
- Ross DD, Karp JE, Chen TT, et al. Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood 2000;96:365-8. [PubMed]
- Yoh K, Ishii G, Yokose T, et al. Breast cancer resistance protein impacts clinical outcome in platinum-based chemotherapy for advanced non-small cell lung cancer. Clin Cancer Res 2004;10:1691-7. [PubMed]
- Diestra JE, Condom E, Del Muro XG, et al. Expression of multidrug resistance proteins P-glycoprotein, multidrug resistance protein 1, breast cancer resistance protein and lung resistance related protein in locally advanced bladder cancer treated with neoadjuvant chemotherapy: biological and clinical implications. J Urol 2003;170:1383-7. [PubMed]
- Usuda J, Tsunoda Y, Ichinose S, et al. Breast cancer resistant protein (BCRP) is a molecular determinant of the outcome of photodynamic therapy (PDT) for centrally located early lung cancer. Lung Cancer 2010;67:198-204. [PubMed]
- Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24:2137-50. [PubMed]
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Ilson DH, Saltz L, Enzinger P, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 1999;17:3270-5. [PubMed]
- Ajani JA, Baker J, Pisters PW, et al. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer 2002;94:641-6. [PubMed]
- Horner MJ, Ries LAG, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2006, National Cancer Institute. Bethesda, MD, Available online: http://seer.cancer.gov/csr/1975_2006/, based on November 2008 SEER data submission, posted to the SEER web site, 2009.
- Olaussen KA, Dunant A, Fouret P, et al. DNA repair by ERCC1 in non-small-cell lung cancer and cisplatin-based adjuvant chemotherapy. N Engl J Med 2006;355:983-91. [PubMed]
- Hoffmann AC, Wild P, Leicht C, et al. MDR1 and ERCC1 expression predict outcome of patients with locally advanced bladder cancer receiving adjuvant chemotherapy. Neoplasia 2010;12:628-36. [PubMed]
- Matsubara J, Nishina T, Yamada Y, et al. Impacts of excision repair cross-complementing gene 1 (ERCC1), dihydropyrimidine dehydrogenase, and epidermal growth factor receptor on the outcomes of patients with advanced gastric cancer. Br J Cancer 2008;98:832-9. [PubMed]
- Handra-Luca A, Hernandez J, Mountzios G, et al. Excision repair cross complementation group 1 immunohistochemical expression predicts objective response and cancer-specific survival in patients treated by Cisplatin-based induction chemotherapy for locally advanced head and neck squamous cell carcinoma. Clin Cancer Res 2007;13:3855-9. [PubMed]
- Hwang IG, Jang JS, Do JH, et al. Different relation between ERCC1 overexpression and treatment outcomes of two platinum agents in advanced biliary tract adenocarcinoma patients. Cancer Chemother Pharmacol 2011;68:935-44. [PubMed]
- Kawato Y, Aonuma M, Hirota Y, et al. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res 1991;51:4187-91. [PubMed]
- Williamson SK, McCoy SA, Gandara DR, et al. Phase II trial of gemcitabine plus irinotecan in patients with esophageal cancer: a Southwest Oncology Group (SWOG) trial. Am J Clin Oncol 2006;29:116-22. [PubMed]
- Lustberg MB, Bekaii-Saab T, Young D, et al. Phase II randomized study of two regimens of sequentially administered mitomycin C and irinotecan in patients with unresectable esophageal and gastroesophageal adenocarcinoma. J Thorac Oncol 2010;5:713-8. [PubMed]


