|
Original Article
Increasing incidence in liver cancer in Canada, 1972–2006:
Age-period-cohort analysis
XiaoHong Jiang1, Sai Yi Pan1, Margaret de Groh1, Shiliang Liu2, Howard Morrison1
1Science Integration Division, 2Health Surveillance and Epidemiology Division, Centre for Chronic Disease Prevention and Control, Public Health Agency
of Canada, Ottawa, Canada
Corresponding author: Dr. Margaret de Groh, Science Integration Division,
Centre for Chronic Disease Prevention and Control, Public Health Agency
of Canada, 9th Floor, Room 926A5, 785 Carling Ave. Ottawa, ON K1A 0K9,
Canada. Tel: 613–957–1786; Fax: 613–941–5497. Email: Margaret.deGroh@phac-aspc.gc.ca
|
|
Abstract
Background/Aims: Our study aimed to assess 1) the temporal trends in incidence and mortality of liver cancer and 2)
age-period-cohort effects on the incidence in Canada.
Methods: We analyzed data obtained from the Canadian Cancer Registry Database and Canadian Vital Statistics Death
Database. We first examined temporal trends by sex, age group, and birth cohort between 1972 and 2006. Three–year period
rates and annual percentage change (APC) were calculated to compare the changes over the study period. We used
age-period-cohort modelling to estimate underlying effects on the observed trends in incidence.
Results: The overall age-adjusted incidence rates increased from 2.6 and 1.5 per 100 000 in 1972–74 to 6.5 (APC: 2.9)
and 2.2 (APC: 1.2) per 100 000 in 2004–06 among males and females, respectively. The age-adjusted mortality rates
increased from 3.3 and 2.0 per 100 000 in 1972–74 to 6.0 (APC: 2.3) and 2.6 (APC: 1.2) per 100 000 in 2004–06 among
males and females, respectively. The incidence increased most rapidly in men aged 45–54 years (APC: 4.1) and women
aged 65–74 years (APC: 1.7) over the period of study.
Conclusions: The age-period-cohort analysis suggests that birth-cohort effect is underlying the increase in incidence.
While the exact reason for the increased incidence of liver cancer remains unknown, reported increase in HBV and HCV
infections, and immigration from high-risk regions of the world may be important factors.
Key words
Incidence of liver cancer, immigration, HBV and HCV, age-period-cohort model
J Gastrointest Oncol 2011; 2: 223-231. DOI: 10.3978/j.issn.2078-6891.2011.024
|
|
Introduction
Liver cancer is the sixth most common cancer worldwide
and the third most common cause of cancer mortality
( 1). Although the incidence of liver cancer is low in North
America and Europe, it is one of the few with an increasing
incidence ( 1-3). The overall incidence to mortality ratio
is near 1 ( 4). Porgnosis is very poor, with a 5-year relative
survival rate of only 18% among Canadians diagnosed between 2004 and 2006 ( 5). As a result, the proportion
of liver cancer death in Canada has increased from 2.1%
(ranked 14 th) of all cancer deaths in 2000 to 2.6% (ranked
9 th) in 2007. Men are more vulnerable to developing liver
cancer than women, with male to female ratios between 2:1
and 4:1 ( 1, 3, 6). Liver cancer has several subtypes, including
hepatocellular carcinoma (HCC), cholangiocarcinoma,
hepatoblastoma, and angiosarcoma. HCC accounts for
between 85% and 90% of all liver cancers, while most of
the remaining liver cancers are cholangiocarcinoma ( 6, 7).
Major risk factors for liver cancer include hepatitis B virus
(HBV) and hepatitis C virus (HCV) infections and alcohol
abuse, which together may be responsible for up to 90% of
incident cases ( 6-8, 9). In this study, we 1) analysed the temporal trends in
incidence of and mortality due to liver cancer in Canada
from 1972 to 2006; 2) examined the changes in incidence
by age at diagnosis, time period and birth cohort; and
used age-period-cohort modelling to assess the potential underlying effects on the incidence.
|
|
Materials and Methods
We obtained incidence data files for 1972–91 from the
National Cancer Incidence Reporting System (NCIRS) and
for 1992–2006 from the Canadian Cancer Registry (CCR),
and mortality data for 1972–2006 from the Canadian Vital
Statistics Death Database. The Health Statistics Division
of Statistics Canada maintains the data used in this study,
and the databases are considered to be very accurate and
reliable. (A detailed description of the registry, including
data sources, methodology and accuracy, is available on
the Statistics Canada website ( 10) and elsewhere ( 11).) A
very small percentage of incidence cases and deaths were
excluded due to their unknown age. All the incidence
records were converted to codes used in International
Classification of Diseases, Ninth Revision (ICD–9) or
International Classification of Diseases for Oncology, Third
Edition (ICD–O–3) ( 12). To assess cause of mortality, we
used codes from the International Statistical Classification of
Diseases and Related Health Problems, Tenth Revision (ICD–
10) ( 13) for deaths since 2000. The mortality data included
liver unspecified cases because the coding for liver cancer
changed slightly (14-16). To examine the trends of liver cancer over the period
of study, we used the codes ICD–9 155, ICD–O–3 C22
and ICD–10 C22 for liver cancer. First, we contrasted the
average 3–year age-adjusted incidence and mortality rates
for the period 1972–74 with that for 2004–06 for men and
women separately. We then compared the incidence and
mortality rates for five specific age groups that is 35–44,
45–54, 55–64, 65–74 and 75–84 years. We evaluated
secular trends in the incidence and mortality of liver cancer
through linear regression models using logarithms of the
annual rates for all ages as well as for the five age groups.
Correspondingly, the annual percent changes (APC)
during the study period were derived from the regression
coefficients of those models. All age-adjusted incidence
and mortality rates were calculated using 1991 Canadian
population serving as the standard.
Analyses integrating age at diagnosis, time period of
diagnosis and birth cohort were conducted separately for
men and women. We grouped age at diagnosis into 5-year
intervals (35–39 years to 80–84 years) and categorized the
period of diagnosis into 5-year intervals from 1972 through
2006 (1972–76 to 2002–06). Corresponding to these age
intervals and time periods, 16 overlapping 10-year birth
cohorts (1888–97 to 1963–72) were derived for the ageperiod-
cohort analysis of the incidence. We thus computed
and plotted the age-specific incidence rates for all the 16 birth cohorts. A Poisson regression model was used to
estimate the age, period and cohort effects; the model
assumes that the number of incident cases follows a Poisson
distribution and that the incidence rates are a multiplicative
function of the included model parameters, making the
logarithm of the rates an additive function of the parameters
( 17-19). For example, the form of the age-period-cohort
model was given by log(dij/pij) = μ + αi + βj + γk
where log (d ij/p ij) is the rate of interest with d ij denoting
the number of the cases in the ith age group and jth period
and p ij is the population at risk in the ith age group and jth
period; α i is the effect of the ith age group; β j is the effect
of jth period category; and γ k is the effect of the kth cohort
category (k = I – i + j when i = 1, 2,…, I). Inherent in the
three-factor age-period-cohort model is the well-known
non-identifiability problem: parameters for age, period and
cohort can not be uniquely estimated because of the exact
linear dependence of the regression variables (cohort =
period − age) ( 20, 21). Although there are several methods
that can deal with the non-identifiability problem, there
is no consensus in the literature as to which method is
optimal. Hence, we selected two-factor models to calculate
the relative risk as the log of regression coefficients by
adjusting for the other factor. To test the effect of birth
cohort and period of diagnosis individually after controlling
for the effect of age, we compared respective two-factor
models with the full model. Parameters of the models were estimated by means of
the maximum likelihood method with SAS procedure
GENMOD (release SAS Enterprise Guide 4, SAS Institute
Inc.). The goodness-of-fit of models was evaluated using the
deviance, defined to be twice the difference between the
maximum achievable log likelihood and the log likelihood
at the maximum likelihood estimates of the regression
parameters. Specific effects (e.g., period effects) were tested
by comparing the difference in deviance between models
with and without a term for the effect.
|
|
Results
A total of 29 489 cases of liver cancer—19 859 (67.3%) in
men and 9630 (32.7%) in women—were registered, and
31 568 deaths from liver cancer were reported in Canada
between 1972 and 2006. The mortality rate exceeded the
incidence rate among females and also in some years among
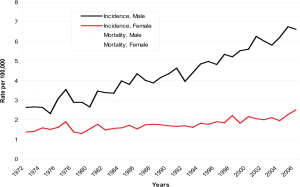
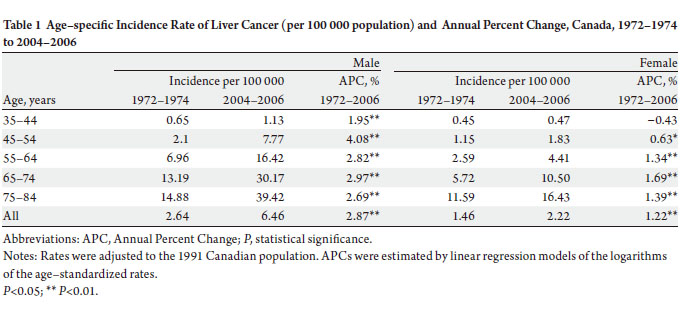
males. The annual age–adjusted incidence rate increased by
145% for men (from 2.64 per 100 000 in 1972–74 to 6.46
per 100 000 in 2004–06) and by 52% for women (from 1.46
per 100 000 in 1972–74 to 2.22 per 100 000 in 2004–06).
Mortality rates showed a similar increase, with the annual age–adjusted rate increasing by 84% (from 3.28 per 100 000
in 1972–74 to 6.02 per 100 000 in 2004–06) for men and
29% (from 2.01 per 100 000 in 1972–74 to 2.59 per 100 000
in 2004–06) for women. This trend appears more marked
among men than women, especially since the early 1990s
( Figure 1). The increase in overall incidence rates of liver cancer
among men was larger than that among women, with an
APC of 2.9% and 1.2%, respectively. Among the respective
age groups, men aged 45–54 years experienced the most
rapid increase in incidence (APC: 4.1%), while women aged
65–74 years had the highest increase (APC: 1.7%) ( Table1).
The increase in mortality among men was higher than that
among women (APC: 2.3% vs. 1.2%). Men aged 75–84 years
had the most rapid increase (APC: 2.8%). Women aged 35–
44 years of age had a statistically significant decrease (APC:
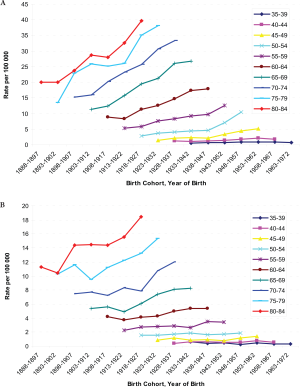
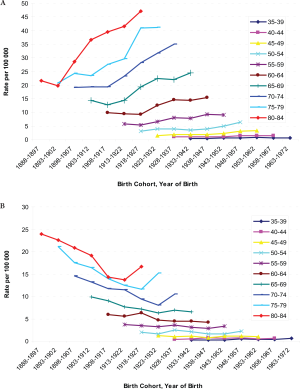
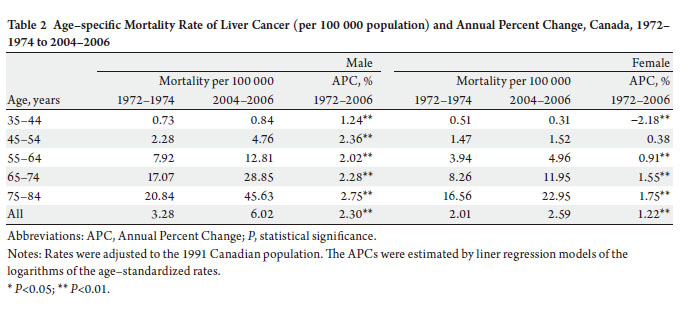
−2.2%) over the study period, however ( Table 2). The age-specific incidence and mortality rates by birth
cohort were plotted in Figure 2 and 3. The incidences
increased as the bir th cohor t advanced, with more
substantial increases in later birth cohorts for both men and
women ( Figure 2a and b). The highest mortality rates in
aged 80–84 years among men, but a decreasing mortality
rate in later birth cohorts in women were observed ( Figure
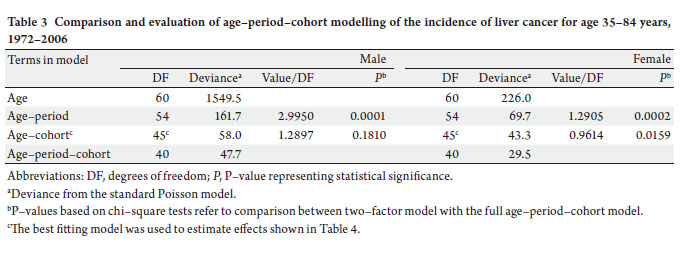
3a and b). The results of fitting age-period-cohort models
to the data are summarized ( Table 3). The birth cohort
effect was statistically significant among men and women;
the period effect was statistically significant among women
only. Further, comparison of the age-period model with
the full age-period-cohort model showed an improvement,
suggesting that the birth-cohort effect was stronger than the
period effect among both men and women. Therefore, we
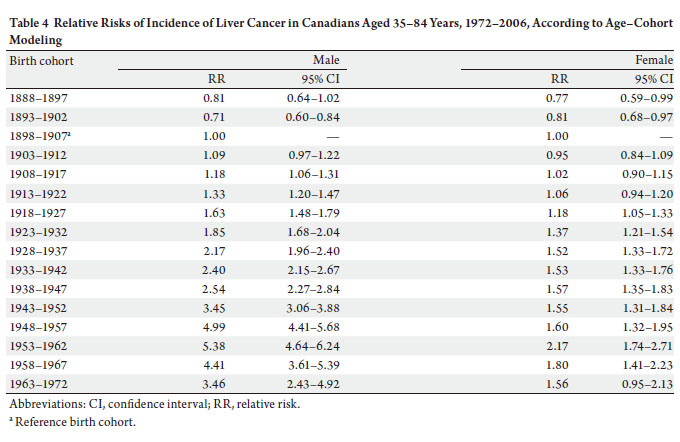
selected the age-cohort model to estimate the relative risk of developing liver cancer. With the incidence rate in birth
cohort 1898–1907 used as the reference, risk of diagnosis
with liver cancer increased in subsequent birth cohorts.
For example, the probability of the birth cohort of 1953–
1962 being diagnosed with liver cancer was over five times
as high for men and two times as high for women compared
with the reference birth cohort of 1898–1907 ( Table 4).
|
|
Discussion
Our data showed that the overall age-adjusted incidence and
mortality rates of liver cancer have increased substantially
since the early 1970s for both men and women in Canada.
The increases were 145% among men and 52% among
women for incidence of liver cancer, and 84% among men
and 29% among women for mortality from liver cancer
between 1972–74 and 2004–06. A limitation of the disease
coding is that mortality data includes liver, unspecified
cases ( 14-16). Our age-period-birth cohort modelling of
the data suggests that birth-cohort effect might have played
an important role in the increase in liver cancer incidence,
although time-period effect could also be involved. Our
results are largely consistent with the reports from Britain,
Italy and the United States ( 1, 2, 22-24). Thus this modeling
indicates that increased exposures to risk factors over time
might be responsible for the increasing incidence of liver
cancer in Canada. The underlying causes could include:
1) change or increase in related conditions such as HBV
and HCV infections and in other risk factors; 2) increase
in immigrant population from high-risk areas such as
Asia and Africa; 3) advances in diagnostic technology and
completeness of registration of cases; and 4) increases
in prevalence of obesity and diabetes mellitus among Canadians. Epidemiological studies found that recent immigrants
from HBV–endemic areas and their descendants were at
high risk of chronic HBV infection and of HBV–related
liver cancer ( 25-27). Immigration from high-risk areas of
hepatitis B infection, drug abuse and needle sharing, blood
transfusion of unscreened blood or blood products, and
unsafe sexual practices in the 1960s and 1970s have been
associated with an increase in the HBV- and HCV- related
liver cancer ( 9, 23, 28, 29). The total number of individuals born outside of Canada
reached 6.2 million, 19.8% of the total population in 2006
( 31). Canadian census data show an increase in immigration from Africa and Asia made up about 17% of the foreignborn
population in 1981, increasing to 28% in 1996 and
42% in 2001. Concurrently, immigrants from Europe made
up a decreasing proportion of the foreign-born population,
beginning at 67% in 1981 and dropping to 55% in 1996
and then 42% in 2001 ( 28). Immigrants from high-risk
areas for HBV infection showed elevated rates of several
diseases including liver cancer ( 27, 29). The highest annual
percentage change (APC) among 45–65 men liver cancer
could be inf luenced by immigration from high-risk areas
of hepatitis B infection. Previous epidemiological studies
associated an increase in immigrants from high-risk areas
with the rise in incidence and mortality of liver cancer in Canada ( 30, 32). Analysis by cultural background and region
of birth revealed a high incidence of and mortality due to
liver cancer for immigrants from certain specific regions.
Chen et al. found that the risk of liver cancer was associated
with a high proportion of immigration to the province of
Ontario ( 28). Luo et al. examined the incidence of cancer
among Chinese immigrants in Alberta and found that the
overall cancer incidence was lower among immigrants,
but the incidence rates of liver cancer were much higher
(16.7/100 000) than that among Canadian-born residents
(1.7/100 000) of Alberta ( 32). The increased incidence
rates of liver cancer observed in those studies were likely to be associated with the high prevalence of HBV and HCV
infections among high-risk groups. Immigrants might
have acquired such infection before coming to Canada.
One study found increased risk among immigrants from
South–Eastern Asia infected with biliary liver flukes where
consumption of raw fresh-water fish is a cultural practice.
Biliary liver fluke has an infrequent cause of infection which
the potential long-term consequences of chronic infection
are highly associated with cholangiocarcinoma ( 33). Liver cancer is more prevalent in men than in women
worldwide ( 1, 2). We observed a male to female ratio of
around 2:1 for liver cancer incidence and mortality in Canada. We also observed that the increasing trends of
incidence and mortality of liver cancer among men started
at 45 years of age. The reasons for higher rates of liver cancer
in males may be due to sex-specific differences in exposure
to risk factors ( 27). Further, epidemiological studies have
indicated that males are more sensitive to the effect of HBV
infection than females. Wang et al. found that there was a
greater risk difference between hepatitis B surface antigen
carriers and noncarriers among males than among females,
and that males had a significant synergistic effect for the
interaction between sex and HBV infection on liver cancer
mortality ( 34). A case-control study by Yu et al. found an
inverse relation between exposure to estrogens and liver
cancer, suggesting that estrogens may provide a protective
effect against liver cancer ( 35). Naugler et al. found that
estrogen-mediated inhibition of interleukin-6 production
by Kupffer cells reduced the risk of liver cancer in females
( 36). Heavy consumption of alcohol is a well-known risk factor
for liver cancer. Donato et al. found a positive linear relation
between the risk of hepatocellular carcinoma (HCC) and
alcohol intake, although these was no substantial relative
risk differences between men and women ( 37). Further,
risk of liver cancer is thought to be affected by synergistic
interactions between HBV or HCV infection and alcohol
( 38). A systematic review of epidemiological evidence concluded that HBV infection, HCV infection and alcohol
intake are major causes of HCC worldwide, and the three
main risk factors together account for approximately 85%
of the total HCC cases ( 39). Boffetta et al. found that DNA
damage occurred after heavy alcohol consumption, and
alcohol-associated liver cirrhosis was the most important
risk factor for HCC in populations with low prevalence of
infection from HBV and HCV, such as in the United States
and northern Europe ( 40). Heavy alcohol consumption
may cause DNA damage by reducing intake of nutrition;
this could also explain the synergistic effect of alcohol and
HBV and HCV infections ( 41). In addition, other studies
also reported a strong association between obesity and
liver cancer, although the mechanisms for this association
remain unknown ( 42, 43). The metabolic abnormalities
related to excess weight include high plasma triglyceride,
glucose and insulin levels, as well as insulin resistance. Period effect identif ied by our age-period-cohort
regression, though statistically significant only in women,
is very likely due to an overall improvement in the quality
of cancer registration data that took place in the 1970s
and early 1980s in most Canadian provinces/territories,
especially Quebec ( 21, 44). Changes to cancer diagnostic
criteria and registration methodology over that period were
already documented by the Canadian Cancer Registry;
however, its impact on the cancer trends was too small to be quantified by earlier studies ( 11, 44). In addition, the
slight decrease in relative risks in the two most recent birth
cohorts ( Table 4) may indirectly indicate a likelihood
that risks for liver cancer attributable to exposure to the
risk factors identified above have yet to appear in younger
generations. In summary, substantial increases in incidence and
mortality from liver cancer have occurred over the last 3
decades, with increases in rates among men over twice that
for women. The underlying birth-cohort pattern observed
for liver cancer incidence in the Canadian population
suggests an increasing proportion of immigrants from highrisk
regions, an increased prevalence of persons infected
with HBV and HCV, and increasing rates of obesity are
responsible for the increasing risk of developing liver cancer.
Direct epidemiological evidence, however, is needed, as
other yet unrecognized etiologic factors may remain to be
identified.
|
|
Acknowledgements
We are grateful to Statistics Canada for access to the
data provided to Public Health Agency of Canada. The
cooperation of the provincial and territorial cancer registries
that supply the data to Statistics Canada is gratefully
acknowledged. The authors particularly thank Robert
Semenciw and Larry Ellison for their critical review of the
manuscript.
|
|
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA
Cancer J Clin 2005;55:74-108.[LinkOut]
- McGlynn K A, Tsao L, Hsing AW, Devesa SS, Fraumeni JF Jr.
International trends and patterns of primary liver cancer. Int J Cancer
2001;94:290-6.[LinkOut]
- Pocobelli G, Cook LS, Brant R, Lee SS. Hepatocellular carcinoma
incidence trends in Canada: analysis by birth cohort and period of
diagnosis. Liver Int 2008;28:1272-9.[LinkOut]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates
of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J
Cancer 2010; 127:2893-917.[LinkOut]
- Ellison LF, Wilkins K. An update on cancer survival. Health Rep
2010;21:55-60.[LinkOut]
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology
and molecular carcinogenesis. Gastroenterology 2007;132:2557-76.[LinkOut]
- McGlynn KA, London WT. Epidemiolog y and natura l histor y
of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol
2005;19:3-23.[LinkOut]
- Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The
contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006;45:529-38.[LinkOut]
- ElSaadany S, Giulivi A. Epidemiology of hepatocellular carcinoma
in Canada, 1995: analysis of death certificates. Chronic Dis Can
2006;27:125-9.[LinkOut]
- Statistics Canada. Canadian Cancer Registr y[Internet]. Health
Statistics Division. 1992[cited 2011 February]. Available from: http://www.statcan.gc.ca/cgi-bin/imdb/p2SV.pl?Function=getSurvey&SDD
S=3207&land=en&db=imdb&adm=8&dis=2.[LinkOut]
- Gaudette L, Lee J, Silberberger C. Cancer: incidence and deaths in
Canada, 1990. Health Rep 1993;5:348-54.[LinkOut]
- Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et
al. International classification of diseases for oncology. 3rd edition.
Geneva: World Health Organization; 2005.
- Slee V. International statistical classification of diseases and related
health Problems, 10th Revision (ICD-10). Geneva: World Health
Organization; 1992.
- World Health Organization. International Classification of Diseases,
Eighth Revision (ICD-8). Geneva: WHO; 1967.
- World Health Organization. International Classification of Diseases,
Ninth Revision (ICD-9). Geneva: WHO; 1977.
- World Health Organization. International Classification of Diseases,
Tenth Revision (ICD-10). Geneva: WHO; 1992.
- Clayton D, Schifflers E. Models for temporal variation in cancer rates. I:
Age-period and age-cohort models. Stat Med 1987; 6:449-67.[LinkOut]
- Clayton D, Schifflers E. Models for temporal variation in cancer rates.
II: Age-period-cohort models. Stat Med 1987;6:469-81.[LinkOut]
- Holford TR. Approaches to fitting age-period-cohort models with
unequal intervals. Stat Med 2006;25:977-93.[LinkOut]
- Holford TR. Understanding the effects of age, period, and cohort on
incidence and mortality rates. Annu Rev Public Health 1991;12:425-57.[LinkOut]
- Liu S, Semenciw R, Waters C, Wen SW, Mery LS, Mao Y. Clues to
the aetiological heterogeneity of testicular seminomas and nonseminomas:
time trends and age-period-cohort effects. Int J Epidemiol
2000;29:826-31.[LinkOut]
- Dal Maso L, Lise M, Zambon P, Crocetti E, Serraino D, Ricceri F, et al.
Incidence of primary liver cancer in Italy between 1988 and 2002: an
age-period-cohort analysis. Eur J Cancer 2008;44:285-92.[LinkOut]
- El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing
increase in the incidence of hepatocellular carcinoma in the United
States: an update. Ann Intern Med 2003;139:817-23.[LinkOut]
- West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the
incidence of primary liver and biliary tract cancers in England and
Wales 1971-2001. Br J Cancer 2006; 94:1751-8.[LinkOut]
- Hislop TG, Teh C, Low A, Li L, Tu SP, Yasui Y, et al. Hepatitis B
knowledge, testing and vaccination levels in Chinese immigrants to
British Columbia, Canada. Can J Public Health 2007;98:125-9.[LinkOut]
- Taylor VM, Tu SP, Woodall E, Acorda E, Chen H, Choe J, et al. Hepatitis
B knowledge and practices among Chinese immigrants to the United
States. Asian Pac J Cancer Prev 2006;7:313-7.[LinkOut]
- El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver
cancer in the United States. Arch Intern Med 2000;160:3227-30.[LinkOut]
- Chen Y, Yi Q , Mao Y. Cluster of liver cancer and immigration: a
geographic analysis of incidence data for Ontario 1998-2002. Int J
Health Geogr 2008;7:28.[LinkOut]
- Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide
incidence and trends. Gastroenterology 2004;127:S5-16.[LinkOut]
- McDermott S, Desmeules M, Lewis R, Gold J, Payne J, Lafrance B, et
al. Cancer incidence among Canadian immigrants, 1980-1998: results
from a national cohort study. J Immigr Minor Health 2011;13:15-26.[LinkOut]
- Statistics Canada[Internet]. Population by immigrant status and period
of immigration, 2006 counts, for Canada, provinces and territories
- 20% sample data[modified 2009 March 27; cited 2011 February].
Available from: http://www12.statcan.ca/census-recensement/2006/
dp-pd/hlt/97-557/T403-eng.cfm?Lang=E&T=403&GH=4&SC=1&S
=99&O=A.[LinkOut]
- Luo W, Birkett NJ, Ugnat AM, Mao Y. Cancer incidence patterns
among Chinese immigrant populations in Alberta. J Immigr Health
2004;6:41-8.[LinkOut]
- Stauffer WM, Sellman JS, Walker PF. Biliary liver flukes
(Opisthorchiasis and Clonorchiasis) in immigrants in the United
States: often subtle and diagnosed years after arrival. J Travel Med
2004;11:157-9.[LinkOut]
- Wang N, Zheng Y, Yu X, Lin W, Chen Y, Jiang Q. Sex-modified effect of
hepatitis B virus infection on mortality from primary liver cancer. Am J
Epidemiol 2009;169:990-5.[LinkOut]
- Yu MW, Yang YC, Yang SY, Cheng SW, Liaw YF, Lin SM, et al.
Hormonal markers and hepatitis B virus-related hepatocellular
carcinoma risk: a nested case-control study among men. J Natl Cancer
Inst 2001;93:1644-51.[LinkOut]
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et
al. Gender disparity in liver cancer due to sex differences in MyD88-
dependent IL-6 production. Science 2007;317:121-4.[LinkOut]
- Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et
al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake
and hepatitis virus infections in men and women. Am J Epidemiol
2002;155:323-31.[LinkOut]
- Nomura H, Kashiwagi S, Hayashi J, Kajiyama W, Ikematsu H, Noguchi
A, et al. An epidemiologic study of effects of alcohol in the liver in
hepatitis B surface antigen carriers. Am J Epidemiol 1988;128:277-84.[LinkOut]
- Alcohol drink ing. Biological data relevant to the evaluation of
carcinogenic risk to humans. IARC Monogr Eval Carcinog Risks Hum
1988;44:101-52.[LinkOut]
- Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol 2006;7:149-56.[LinkOut]
- Polesel J, Zucchetto A, Montella M, Dal Maso L, Crispo A, La Vecchia
C, et al. The impact of obesity and diabetes mellitus on the risk of
hepatocellular carcinoma. Ann Oncol 2009;20:353-7.[LinkOut]
- Marrero JA, Fontana RJ, Fu S, Conjeevaram HS, Su GL, Lok
AS. Alcohol, tobacco and obesity are synergistic risk factors for
hepatocellular carcinoma. J Hepatol 2005;42:218-24.[LinkOut]
- Pan SY, Johnson KC, Ugnat AM, Wen SW, Mao Y; Canadian Cancer
Registries Epidemiology Research Group. Association of obesity and
cancer risk in Canada. Am J Epidemiol 2004;159:259-68.[LinkOut]
- Gaudette LA, Lee J. Cancer incidence in Canada, 1969-1993. Ottawa:
Statistics Canada Marketing Department, Minister of Industry; 1997.
Cite this article as:
Jiang X, Pan S, Groh M, Liu S, Morrison H. Increasing incidence in liver cancer in Canada, 1972–2006:
Age-period-cohort analysis. J Gastrointest Oncol. 2011;2(4):223-231. DOI: 10.3978/j.issn.2078-6891.2011.024
|