
Survival and prognostic factors analysis of 151 intestinal and pancreatic neuroendocrine tumors: a single center experience
Introduction
Neuroendocrine tumors (NETs) are rare tumors, with an incidence of 5 per 100,000 persons per year. Intestinal and pancreatic NETs (IP-NETs) represent about 25% of all NETs (1). As they derive from enterochromaffin cells of the neuroendocrine system, they share some common histological characteristics. However, in terms of hormonal secretion, tumor aggressiveness, local and metastatic evolution, response to treatments and prognosis, NETs form a very heterogeneous group.
Tumor grade, stage, degree of differentiation, and primary tumor size, are well recognized as prognostic factors in NETs. Other factors related to poorer prognosis have been suggested like advanced age at diagnosis, pancreatic tumor localization, the presence of synchronous metastases, and functional character of the tumor, but are still discussed (2-13). Tumor grade, evaluated according to the 2006 ENETS classification based on two proliferative markers [mitotic index (MI) and Ki67 labeling index], is considered the major prognostic factor. According to this classification, tumors are classified in 3 groups: grade 1 tumors: MI <2 and Ki67 ≤2%, grade 2 tumors: MI 2-20 and Ki67 ≥2–20%, and grade 3 tumors: MI >20 and Ki67 ≥20%. Five-year survival of IP-NETs is over 90% for grade 1 tumors, but is lower than 50% for grade 3 tumors. Recently, a new WHO 2017 classification for neuroendocrine pancreatic neoplasms has been introduced, in which two types of grade 3 NETs have been distinguished: (I) well differentiated NETs with Ki67 ≥20%, and (II) poorly differentiated (small or large cell) neuroendocrine carcinomas (14).
The clinical management of NETs is currently guided by different factors related to the patient (age, co-morbidities), and to the tumor (tumor grade, size, degree of tumor differentiation, the presence of synchronous metastases, location of metastases, existence of functional syndrome and tumor resectability). The dynamics of tumor evolution over the time, evaluated by tumor progression after 3 to 6 months, is also taken into account (15,16). Given the relative rarity of these tumors and their great heterogeneity, the therapeutic guidelines for the treatment of IP-NETS remain unclear and are frequently open to clinician judgment. The better knowledge of prognostic factors appears to be essential to help the clinician in the choice of the therapeutic strategy adapted to the aggressiveness of the disease.
The aim of our study was to describe clinical and pathological features determine the clinical, therapeutic and pathological factors which influence the survival in IP-NETs.
Methods
Patients
All the patients diagnosed with well differentiated IP-NETs at the Nantes University Hospital between October 1994 and October 2013, were included. Patients with MEN-1 (Type 1 Multiple Endocrine Neoplasia) and Von Hippel-Lindau syndrome were excluded. The data were collected retrospectively, from medical records, or, if necessary, through the direct telephone contact with the patient or with his family doctor. Moreover, the evaluation of MI and Ki67 index of the tumors was performed prospectively, on tumor samples retrieved prospectively from pathology tissue collection, whenever this information was not available in medical files.
Clinical data and tumor properties
Data regarding first symptoms leading to diagnosis, type of tumor, tumor location, tumor size, presence of metastases at diagnostic or during evolution and metastasis location, were collected.
Histological characteristics
Tumor tissues, of primary tumor or metastases or both when available, were analyzed prospectively by an expert pathologist. MI and Ki67 labeling index were analyzed to determine tumor grade according to the WHO 2010 classification. Ki67, a nuclear marker of cells in active phase of the cell cycle (G1, S1, G2 and mitosis), was analyzed on 2,000 tumor cells in areas of highest nuclear labelling index and expressed as percentage of stained cells. MI was evaluated according to a standard method after hematoxylin and eosin saffron staining, and additionally by using immunostaining with phosphohistone H3 (PPH3)- antibody, which stains the late-G2 and M phases of mitosis. Results were expressed by the number of mitoses per 10 fields. The “global grade” was the highest grade between primary tumor and metastasis.
Statistical analysis
Survival rates were assessed by Kaplan-Meier method. Global survival was determined as the time between obtention of the histological proof of tumor and death from any cause or date of the last news. Differences in median survival were compared using the log-rank test. Cox regression models were used for determination of independent prognostic factors, by univariate analyses. Variables with a P value <0.05 in univariate analysis were included in the multivariate analysis, performed with the Cox proportional hazard model, with a significance level of P<0.05. Analyses were performed using the Graph Pad Prism 6 and XLStat 2017 software.
Ethics
This is a non-interventional study. This study protocol was conform to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and has been approved by the local Ethic Committee of Nantes University Hospital, France. A consent form was not required for this study.
Results
Patients’ characteristics and treatments
One hundred and fifty-one patients [82 men (54.3%), median age 60 years (range, 14–81)], were included. Among them, 86 patients (56.95%) had primary pancreatic tumor, and 65 patients (43.05%) had primary intestinal tumor. Fifty-two patients (34.4%) had functional tumors. Seventy-two patients had synchronous or metachronous metastases and from them, 27 (37.5%) had a metastases resection (10 with pancreatic primary tumor and 17 with intestinal primary tumor). One hundred and thirty-nine patients (92.05%) had surgery, either of their primary tumor (n=139) or of their metastases (n=27). If complementary treatment was required, this treatment comprised chemotherapy (n=34), transarterial chemoembolization (n=21), targeted radiotherapy (n=13), somatostatin analogues (n=53), and other treatments (n=16). The mean number of treatment lines was 0.7, median 0 (range, 0–7) (Table 1).
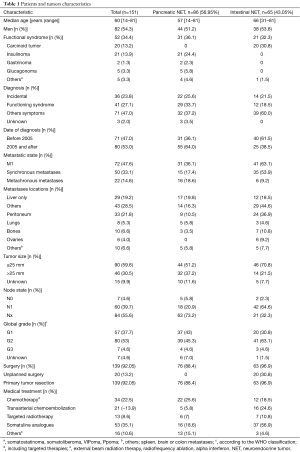
Full table
All IP-NETs
In univariate analysis, the median overall survival (OS) was 157 months. Age >65 years (HR =3.46; 95% CI, 2.45–7.61; P<0.0001), I-NET (HR =2.16; 95% CI, 1.30–3.70; P=0.0035), synchronous metastases (HR =2.61; 95% CI, 1.71–5.43; P=0.0002), ovarian metastasis (HR =2.68; 95% CI, 1.21–20.46; P=0.03), and emergency surgery (HR =2.77; 95% CI, 1.89–10.95; P=0.0008), were identified as statistically significant poor prognostic factors. A Ki67 >5% had a negative impact on survival (HR =2.77; 95% CI, 1.89–4.09; P=0.006), whereas no statistically significant impact was found for a Ki67 ≥2%.
In multivariate analysis, age >65 years old (HR =3.87; 95% CI, 2.19–6.83; P<0.0001), Ki67 >5% (HR =2.43; 95% CI, 1.08–5.46; P=0.03), synchronous metastases (HR =2.37; 95% CI, 1.17–4.76; P=0.016), primary tumor size >25 mm (HR =1.96; 95% CI, 1.08–3.54; P=0.03) and emergency surgery (HR =2.64; 95% CI, 1.30–5.36; P=0.007) were independent poor prognostic factors (Table 2).
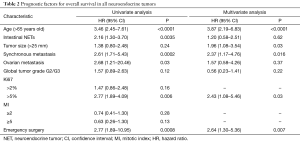
Full table
Non-metastatic pancreatic NETs (n=71)
In univariate analysis, OS was higher for insulinomas as compared to other tumors [HR =0.19; 95% CI, 0.11–1.12; P=0.08). Ki67 >5% (HR =3.43; 95% CI, 1.41–14.57; P=0.013), and age >65 years (HR =8.88; 95% CI, 3.93–39.36; P<0.0001), were poor prognostic factors.
In multivariate analysis, age >65 years (HR =13.62; 95% CI, 2.99–62.04; P=0.001) and Ki67 >5% (HR =4.88; 95% CI, 1.58–15.02; P=0.006) were the only independent poor prognostic factors (Table 3).
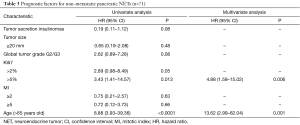
Full table
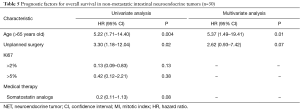
Pancreatic NET’s with synchronous metastases (n=15)
In univariate analysis, age >60 years and OMS score ≥2 were significant poor prognostic factors, but only borderline significant in multivariate analysis with HR =6.09 (95% CI, 0.92–40.50; P=0.06) and HR =5.47 (95% CI, 0.99–30.34; P=0.052), respectively (Table 4).
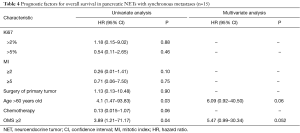
Full table
Non metastatic intestinal NET (n=30)
In univariate analysis, age >65 years (HR =5.22; 95% CI, 1.71–14.40; P=0.004) and unplanned surgery (HR =3.30; 95% CI, 1.18–12.04; P=0.02) were poor prognostic factor.
In multivariate analysis, only age >65 years old (HR =5.37; 95% CI, 1.49–19.41; P=0.01) was an independent prognostic factor (Table 5).
Full table
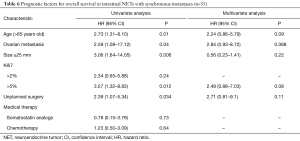
Intestinal NET with synchronous metastases (n=35)
In univariate analysis, age >65 years old (HR =2.73; 95% CI, 1.31–8.10; P=0.01), ovarian metastases (HR =2.59; 95% CI, 1.08–17.12; P=0.04), Ki67 >5% (HR =3.27; 95% CI, 1.32–8.83; P=0.012) and unplanned surgery (HR =2.39; 95% CI, 1.07–5.34; P=0.034) were poor prognostic factors.
In multivariate analysis, no significant independent prognostic factor was identified (Table 6).
Full table
Discussion
In the present study, we analysed the data from 151 patients with IP-NETs, diagnosed and treated in a single center. As IP-NETs are rare and frequently have slow progression, the data were collected retrospectively from 1994 until 2013. We report a large cohort, with a long follow-up. The median survival time for all patients was 161 months. Demographic characteristics and prognostic factors of survival identified in our study are in accordance with those previously reported. In univariate analysis, P-NETs had a better prognosis than I-NETs. In the literature, tumor primary site has been proved to affect OS, with a better outcome for I-NETS (5,17-19). The different outcome observed in our study may be in part related to the high proportion of insulinomas, known for a better prognosis, included in our study (24% of P-NETs), and to a selection bias in a tertiary center expert in pancreatic surgery. In multivariate analysis, for all IP-NETs, the age superior to 65 years, Ki67 proliferation index superior to 5%, the primary tumor size superior to 25 mm, the presence of synchronous metastases and emergency surgery for acute complications, were significant independent poor prognostic factors.
Age at diagnosis appears to be a strong prognostic factor, as previously reported (17,20,21). It could be explained in part by patients’ comorbidities and deaths from other causes than the tumor. However, in patients older than 75 years old, none received more than 1 line of treatment for their IP-NET. Elderly patients may be undertreated, and it could have a significant impact on survival.
Tumor grade, according to the WHO classification, is an established strong prognostic factor (14). In our study, tumor grade was confirmed in a prospective manner, after a systematic analysis of tumor tissue to determine the Ki67, even for NETs diagnosed before 2005. Tumor grade depends on mitotic counts and Ki67. Those 2 proliferative markers are continuous values, but cuts off of 2 and 20 are used to grade the tumor in current guidelines (15,22). In clinical practice, grade 2 NETs form a very heterogeneous group and survival in this group can be very different between a “low” grade 2 with a Ki67 close to 2% or a “high” grade 2 with a Ki67 close to 20%. In our study, a cut off of 5% turned out to be more relevant than a cut off of 2% for Ki67, which has already been suggested (3,6,23). The WHO classification of NET should therefore probably be revised. There are several biomarkers and genetic markers currently under study which can become prognostic markers in the future, but cannot be recommended in clinical practice yet (24-26).
For I-NETs, the resection of primary tumor is generally advocated (ESMO and ENETS guidelines) (27,28). The absence of primary tumor resection can lead to acute complications, such as digestive occlusion, perforation or intestinal ischemia requiring emergency surgery, which impacts the OS. Primary tumors should be resected early to avoid acute complications. In the subgroup of metastatic pancreatic NET in our study, the benefits of primary tumor resection could not be demonstrated, may be due to the small sample size of this population. In other retrospective studies, the surgical resection of the primary tumor in metastatic P-NET was associated with better cancer-specific and OS (29,30). Primary tumor resection could also enhance the response to further treatments, such a peptide receptor radionuclide therapy (PRRT), and improve progression free survival (31).
The survival benefits of the surgical resection of metastases are still controversial (32-36). In our study, there was no impact on survival of surgical resection of metastasis, for all NETS, as well as in P-NETs and I-NETs subgroups. No impact on OS of chemotherapies and targeted therapies were found. But the follow-up since 2007 and the beginning of use of modern chemotherapies and targeted therapies is probably not long enough to find a significant effect on global survival and 5-year survival for these indolent tumors.
There are several limitations to our study. This is a retrospective study, with patients including over a long period. Thus, all the patients could not have been treated with the most recent therapies, targeted therapies or PRRT, which can impact OS. All the NET subgroups were not equally represented, as most of the patients were referred for the resection of P-NETs. Other factors such as comorbidities, smoking and alcohol consumption, and laboratory results could not be documented in our study. However, tumor tissue was analysed prospectively by an expert pathologist.
In conclusion, despite these limitations, we report a large series of patients with IP-NET treated in a single center and identify strong prognostic factors that could help to define therapeutic strategies.
Acknowledgements
This study has been supported by an unrestricted grant from NOVARTIS.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This is a non-interventional study. This study protocol was conform to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008) and has been approved by the local Ethic Committee of Nantes University Hospital, France. A consent form was not required for this study.
References
- Dasari A, Shen C, Halperin D, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Birnbaum DJ, Turrini O, Ewald J, et al. Pancreatic neuroendocrine tumor: A multivariate analysis of factors influencing survival. Eur J Surg Oncol 2014;40:1564-71. [Crossref] [PubMed]
- Chen L, Zhou L, Zhang M, et al. Clinicopathological features and prognostic validity of WHO grading classification of SI-NENs. BMC Cancer 2017;17:521. [Crossref] [PubMed]
- Gao H, Liu L, Wang W, et al. Novel recurrence risk stratification of resected pancreatic neuroendocrine tumor. Cancer Lett 2018;412:188-93. [Crossref] [PubMed]
- Merola E, Rinzivillo M, Cicchese N, et al. Digestive neuroendocrine neoplasms: A 2016 overview. Dig Liver Dis 2016;48:829-35. [Crossref] [PubMed]
- Jin K, Luo G, Xu J, et al. Clinical outcomes and prognostic factors of resected pancreatic neuroendocrine neoplasms: A single-center experience in China. Oncol Lett 2017;13:3163-8. [Crossref] [PubMed]
- Panzuto F, Boninsegna L, Fazio N, et al. Metastatic and locally advanced pancreatic endocrine carcinomas: analysis of factors associated with disease progression. J Clin Oncol 2011;29:2372-7. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after « carcinoid »: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Zhang M, Zhao P, Shi X, et al. Clinicopathological features and prognosis of gastroenteropancreatic neuroendocrine neoplasms in a Chinese population: a large, retrospective single-centre study. BMC Endocr Disord 2017;17:39. [Crossref] [PubMed]
- Panzuto F, Merola E, Rinzivillo M, et al. Advanced digestive neuroendocrine tumors: metastatic pattern is an independent factor affecting clinical outcome. Pancreas 2014;43:212-8. [Crossref] [PubMed]
- Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1-18. vii. [Crossref] [PubMed]
- Ekeblad S, Skogseid B, Dunder K, et al. Prognostic factors and survival in 324 patients with pancreatic endocrine tumor treated at a single institution. Clin Cancer Res 2008;14:7798-803. [Crossref] [PubMed]
- Janson ET, Holmberg L, Stridsberg M, et al. Carcinoid tumors: analysis of prognostic factors and survival in 301 patients from a referral center. Ann Oncol 1997;8:685-90. [Crossref] [PubMed]
- Lloyd RV, Osamura RY, Klôppel G, et al. WHO Classification of Tumours of Endocrine Organs. Available online: http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Who-Classification-Of-Tumours-Of-Endocrine-Organs-2017
- Pavel M, de Herder WW. ENETS Consensus Guidelines for the Standard of Care in Neuroendocrine Tumors. Neuroendocrinology 2017;105:193-5. [Crossref] [PubMed]
- Pavel M, O’Toole D, Costa F, et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016;103:172-85. [Crossref] [PubMed]
- Panzuto F, Nasoni S, Falconi M, et al. Prognostic factors and survival in endocrine tumor patients: comparison between gastrointestinal and pancreatic localization. Endocr Relat Cancer 2005;12:1083-92. [Crossref] [PubMed]
- Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis of 146 metastatic neuroendocrine tumors of the mid-gut. Neuroendocrinology 2009;89:471-6. [Crossref] [PubMed]
- Strosberg J, Gardner N, Kvols L. Survival and prognostic factor analysis in patients with metastatic pancreatic endocrine carcinomas. Pancreas 2009;38:255-8. [Crossref] [PubMed]
- Lepage C, Ciccolallo L, De Angelis R, et al. European disparities in malignant digestive endocrine tumours survival. Int J Cancer 2010;126:2928-34. [PubMed]
- Landerholm K, Zar N, Andersson RE, et al. Survival and prognostic factors in patients with small bowel carcinoid tumour. Br J Surg 2011;98:1617-24. [Crossref] [PubMed]
- Perren A, Couvelard A, Scoazec JY, et al. ENETS Consensus Guidelines for the Standards of Care in Neuroendocrine Tumors: Pathology: Diagnosis and Prognostic Stratification. Neuroendocrinology 2017;105:196-200. [Crossref] [PubMed]
- Nuñez-Valdovinos B, Carmona-Bayonas A, Jimenez-Fonseca P, et al. Neuroendocrine Tumor Heterogeneity Adds Uncertainty to the World Health Organization 2010 Classification: Real-World Data from the Spanish Tumor Registry (R-GETNE). The Oncologist 2018;23:422-32. [Crossref] [PubMed]
- Ohmoto A, Rokutan H, Yachida S. Pancreatic Neuroendocrine Neoplasms: Basic Biology, Current Treatment Strategies and Prospects for the Future. Int J Mol Sci 2017;18. [Crossref] [PubMed]
- Cen D, Chen J, Li Z, et al. Prognostic significance of cytokeratin 19 expression in pancreatic neuroendocrine tumor: A meta-analysis. PloS One 2017;12:e0187588. [Crossref] [PubMed]
- Luo G, Jin K, Cheng H, et al. Carbohydrate antigen 19-9 as a prognostic biomarker in pancreatic neuroendocrine tumors. Oncol Lett 2017;14:6795-800. [PubMed]
- Partelli S, Bartsch DK, Capdevila J, et al. ENETS Consensus Guidelines for Standard of Care in Neuroendocrine Tumours: Surgery for Small Intestinal and Pancreatic Neuroendocrine Tumours. Neuroendocrinology 2017;105:255-65. [Crossref] [PubMed]
- Öberg K, Knigge U, Kwekkeboom D, et al. ESMO Guidelines Working Group. Neuroendocrine gastro-entero-pancreatic tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23:vii124-30. [PubMed]
- Bertani E, Fazio N, Botteri E, et al. Resection of the primary pancreatic neuroendocrine tumor in patients with unresectable liver metastases: possible indications for a multimodal approach. Surgery 2014;155:607-14. [Crossref] [PubMed]
- Tao L, Xiu D, Sadula A, et al. Surgical resection of primary tumor improves survival of pancreatic neuroendocrine tumor with liver metastases. Oncotarget 2017;8:79785-92. [Crossref] [PubMed]
- Bertani E, Fazio N, Radice D, et al. Resection of the Primary Tumor Followed by Peptide Receptor Radionuclide Therapy as Upfront Strategy for the Treatment of G1-G2 Pancreatic Neuroendocrine Tumors with Unresectable Liver Metastases. Ann Surg Oncol 2016;23:981-9. [Crossref] [PubMed]
- Ahmed A, Turner G, King B, et al. Midgut neuroendocrine tumours with liver metastases: results of the UKINETS study. Endocr Relat Cancer 2009;16:885-94. [Crossref] [PubMed]
- Touzios JG, Kiely JM, Pitt SC, et al. Neuroendocrine hepatic metastases: does aggressive management improve survival? Ann Surg 2005;241:776-83; discussion 783-5. [Crossref] [PubMed]
- Cusati D, Zhang L, Harmsen WS, et al. Metastatic nonfunctioning pancreatic neuroendocrine carcinoma to liver: surgical treatment and outcomes. J Am Coll Surg 2012;215:117-24; discussion 124-5. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [Crossref] [PubMed]
- Watzka FM, Fottner C, Miederer M, et al. Surgical therapy of neuroendocrine neoplasm with hepatic metastasis: patient selection and prognosis. Langenbecks Arch Surg 2015;400:349-58. [Crossref] [PubMed]


