Gastric squamous cell carcinoma and gastric adenosquamous carcinoma, clinical features and outcomes of rare clinical entities: a National Cancer Database (NCDB) analysis
Introduction
Gastric adenocarcinoma (GAC) is the most common primary gastric cancer. Gastric squamous cell carcinoma (GSCC) accounts for 0.2% of primary gastric cancers (1,2). Published literature on GASC and GSCC is limited to case reports and institutional case series. Few reports analyzed published cases (3,4). GSCC is more common in men, usually presents at late stages, and there is no standard treatment approach for this rarely encountered clinical entity. GASC is more common in men, Asians, and incidence peeks in the 6th decade of life. Clinically it is frequently located in proximal stomach, and the prognosis is often based on the adenocarcinoma component (5,6).
The origin of GSCC is unknown and postulated sites include: gastric vessel endothelium, undifferentiated basal stem cells, pre-existing heterotopic squamous epithelium, squamous metaplasia from gastric mucosa due to chronic inflammation, or GSCC replacing an original adenocarcinoma (7,8). EBV infection has been proposed as a possible cause of GSCC based on a case report with documented EBV in the tumor specimen by PCR testing (8). Pathological diagnostic criteria for squamous lesions in the stomach include: presence of keratinizing cell masses with typical pearl formation; mosaic pattern of cell arrangement with early pearl formation; intercellular bridges; presence of high concentrations of sulfhydryl and/or disulfide groups, indicating the presence of keratin or prekeratin (9). In addition to the pathologic features, the Japanese Gastric Cancer Association criteria for primary GSCC require presence of normal gastric mucosa between the gastroesophageal junction and the GSCC lesion (3,10). These criteria ensure that GSCC arising in the cardia is a primary gastric tumor distinct from local extension of esophageal squamous cell cancer. These criteria address earlier concerns raised by Parks et al. regarding the origin of GSCC (11). With the advancement of endoscopic, cross sectional imaging and pathological techniques identification of GSCC tumors as a unique entity and not an extension of esophageal squamous cell cancers has become clinically feasible. In the case series by Boswell and Helwig (9), pure GSSC patients were predominantly observed in male and patients tended to live less than 7 months. The patients with GASC in the same case series tended to be predominantly male, with age less than 60 years, and have primary lesion in the pylorus.
Diagnosis of GASC requires coexistence of both adenocarcinoma and squamous cell carcinoma in the primary tumor and squamous component should exceed 25% of the primary tumor (12,13). Several hypotheses have been proposed for pathogenesis of GASC so far including squamous metaplastic transformation of adenocarcinoma; transformation of ectopic squamous epithelium or transformation of metaplastic squamous cells; collision of concurrent AC and SCC (5), or stem cells differentiation toward both glandular and squamous cells (7,14). GAC accounts for about 95% of all gastric cancers and 5-year overall survival is 20–30% (2).
In the setting of limited published literature on GSCC and GASC, clinical management is based on small single institutional case reports or series. In order to better characterize these rare tumors and evaluate their outcome, a comparison of GSCC, GASC and GAC was performed using the National Cancer Database (NCDB) between 2004 and 2013. The primary objective was to compare the clinical baseline criteria and overall survival of patients with GSCC, GASC and GAC.
Methods
Patient selection
NCDB is a national cancer directory that represents approximately 70% of all newly diagnosed cancers in the US. Inclusion criteria included the following ICD-O-3 [International Classification of Diseases for Oncology, third edition (ICD-O-3) morphological codes (8070/3, 8560/3 and 8140/3)] and topography codes (C16.0-9) in participant user data file between years 2004 and 2013. Patients diagnosed in years 2004–2014 were available in the dataset. Patients who were diagnosed in 2014 were excluded because their survival information was not available. Exclusion criteria are: patients with non-invasive tumors, gastric cancer not the first primary malignancy, didn’t receive any treatment at the reporting facility (no treatment information available), missing survival outcome. Patient-specific data included age, gender, race, histology, insurance status, presence of metastatic disease and co-morbid medical conditions, year of diagnosis and location where treatment was received. The primary outcome was overall survival of patients with GAC, GASC, and GSCC, defined as months between date of diagnosis and date of death or end of follow-up. Ethical approval was not required for the study since patient information in the database is completely de-identified and accessible to the public
Statistical analysis
The descriptive statistics for clinical and demographic characteristics were summarized by mean and standard deviations for numeric variables, numbers and percentages for categorical variables. Univariate association between histology and other covariates were assessed by Chi-square tests for categorical data, and ANOVA for numeric data. Cox proportional hazard models were conducted in univariate and multivariate settings to evaluate the association between patient characteristics (same set of covariates as in the descriptive analyses) and overall survival. Kaplan-Meier plots were generated with log-rank test to compare the survival curves of each histology group. All analyses were done using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina, USA) with a significant level of 0.05.
Results
Baseline patient characteristics
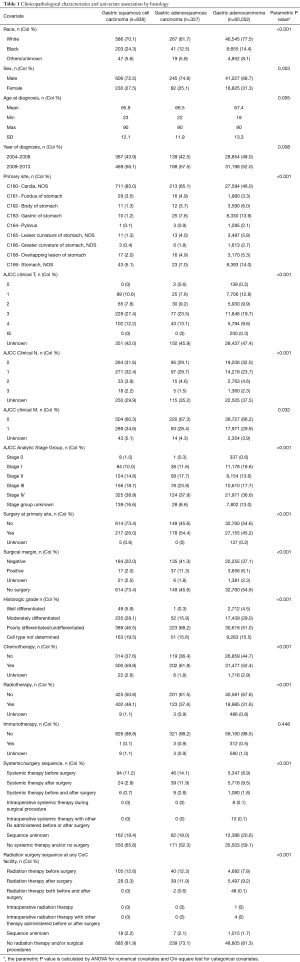
A total of 61,215 patients with gastric malignancies met the inclusion and exclusion criteria. Of those, the majority were GAC (60,052 or 98.1%), followed by GSCC (836 or 1.4%) and GASC (327 or 0.5%). Clinicopathological baseline characteristics of the patients are listed in Table 1. The majority of the patients were men (GSCC 72.5%, GASC 74.9% and GAC 68.7%) and Caucasian (GSCC 70.1%, GASC 81.7% and GAC 77.5%). A statistically significant difference was observed for the location of the primary tumor with GSCC 85.0% in gastric cardia followed by GASC 65.1%, and GAC 46.0%. Potentially resectable disease (stage I, II and III) were significantly different across the three groups with the lowest being GSCC (43.5%) followed by GASC (53.2%) and GAC (49.8%). 38.5%, 35.8% and 30.5% of GSCC, GASC and GAC patients had clinically lymph node positive disease. Poorly differentiated or undifferentiated histology was seen in 46.5%, 68.2%, 51.0% of GSCC, GASC, and GAC patients.
Full table
Treatment patterns
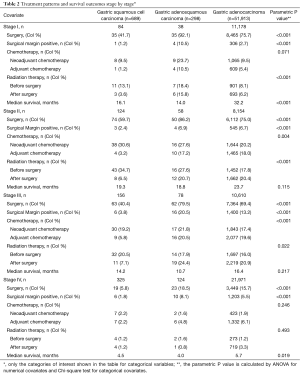
Surgical resection was performed in 26.0%, 54.4% and 45.2% of GSCC, GASC and GAC patients. Surgical margins were positive in 2.0%, 11.3% and 6.1%, respectively. Majority of the patients were treated with systemic chemotherapy, 59.8%, 61.8% and 52.4% of patients with GSCC, GASC and GAC, 11.2%, 14.1% and 8.9% of patients with GSCC, GASC, and GAC underwent neoadjuvant chemotherapy. 2.9%, 11.9%, 9.5% of patients with GSCC, GASC, GAC underwent adjuvant chemotherapy. Radiotherapy was administered in 48.1%, 37.6% and 31.6% of patients with GSCC, GASC and GAC. Radiation therapy was administered before surgery in 12.6%, 12.3%, 7.8% and in 3.3% 11.9%, 9.2% after surgery in GSCC, GASC, and GAC patients. Sequence of radiation therapy was unknown in approximately 2% of all histology types. Small number of patients was treated with immunotherapy, 0.1%, 0.9% and 0.5% in GSCC, GASC and GAC. For patients who had analytical staging data the treatment patterns of all three histology types are outlined in the Table 2. Of the patients with stage II and III GSCC, GASC, GAC neoadjuvant chemotherapy use was 30.6%, 27.6%, 20.2% and 19.2%, 21.8%, 17.4%, respectively. Of the patients with stage II and III GSCC, GASC, GAC surgery was performed in 59.7%, 86.2%, 75.0% vs. 40.4%, 79.5%, 69.4%. Margin positivity was 2.4%, 6.9%, 6.7% for stage II GSCC, GASC, GAC versus 3.8%, 20.5%, 13.2% in stage III. Adjuvant chemotherapy was used in 3.2%, 17.2%, 18.0% of stage II GSCC, GASC, GAC and 5.8%, 20.5%, 19.6% in stage III. Radiation therapy was administered in 34.7%, 27.6%, 17.8% before surgery in stage II GSCC, GASC, GAC and 20.5%, 17.9%, 16.0% in stage III. Postoperative radiation therapy was administered in 6.5%, 20.7%, 20.4% stage II GSCC, GASC, GAC and 7.1%, 24.4%, 20.9% in stage III. Of the patients with stage IV GSCC, GASC, GAC 5.8% (n=19), 18.5% (n=23) and 15.7% (n=3,449) underwent surgery with positive margin rate of 1.8% (n=6), 8.1% (n=10), and 5.5% (n=1,203).
Full table
Outcomes
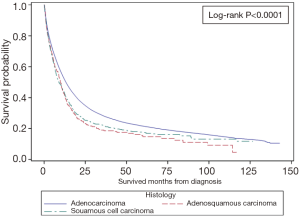
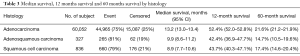
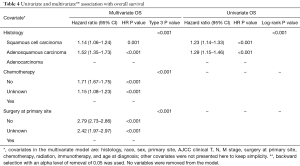
Among all patients GAC had the best median OS (13.2 months) compared to GSSC (8.9 months) and GASC (9.9 months) (Figure 1). One-year OS was 43.7%, 42.4%, and 52.4%, whereas 5-year OS was 17.4%, 14.7% and 21.6% for GSCC, GASC, and GAC (Table 3). Univariate Cox proportional hazard model revealed that squamous cell histology (HR =1.23; 95% CI, 1.14–1.33; P<0.001) and adenosqumaous cell histology (HR =1.29; 95% CI, 1.15–1.46; P=0.001) was associated with worse survival compared to adenocarcinoma histology (Table 4). On multivariate analysis squamous cell (HR =1.14; 95% CI, 1.06–1.24; P=0.001) and adenosquamous cell histology (HR =1.52; 95% CI, 1.35–1.73; P<0.001) was associated with worse survival compared to adenocarcinoma histology (Table 4). In the entire cohort not receiving chemotherapy or surgery was associated with worse survival (HR =1.71; 95% CI, 1.67–1.75; P<0.001 and HR =2.79, 95% CI: 2.73–2.86; P<0.001, respectively) (Table 4). When compared stage by stage median OS was worse in stage I, II, III, IV GASC patients compared to same stage GSCC, and GAC (Table 2).
Full table
Full table
Discussion
GSCC and GASC are rare tumors with unique clinicopathological characteristics compared to GAC. The aim of this study is to improve the current understanding of epidemiology, clinical presentation, pathological features, multimodality treatment utilization, and overall survival of the rare clinical entities GSCC and GASC and provide a comparison to GAC. Various reports provided different incidences, therapies and clinical outcomes. Thomas et al. reported 0.8% of all gastric cancers were GSCC in the surveillance, epidemiology, and end results program (SEER) database analysis from 1973–1987, and these cases included gastric cardia tumors (15). In this analysis of NCDB 1.4% of all cases were GSCC, which may be related to improved diagnostic and endoscopic techniques. In comparison to GAC, GSCC is more common in men and over age 60 years. GSCC tends to originate in the proximal stomach and present at advanced stage. These findings parallel prior reports (1,3,16). Recent results from a SEER database study, identified 163 cases of GSCC (1). In comparison to GAC GSCC presented with stage IV disease (47.2%), had poorly differentiated histology (58.9%) and mean age was 69.6 (range, 33–93) years, man to woman ratio was 2.3:1. More patients with GSCC were African American (24.3%) compared to GAC (14.4%).
GASC is reported to be less than 0.5% of all primary gastric cancers which is similar to the present study (17). Clinical features and outcomes of 167 GASC cases were reported (18). Only 109 cases with R0 resection were included in survival analysis. In that study, 57.8% of patients were >60 years of age, 73.3% were male, 25.4% had metastatic disease, 45% of tumors were located in lower third of stomach, 52.7% had T4 disease, 86.2% has lymph node metastasis. In the contrary the GASC cases in the present study were predominantly located in the cardia and had less T4 disease, and 37.9% had metastatic disease. GAC in Eastern Asian populations is usually intestinal type and more distally located, however in Western populations like US it tends to be diffuse infiltrative type and more proximally located.
There is no standard of care of GSCC and GASC. Surgical resection remains the most commonly used treatment modality in the literature despite about half of the patients with GSCC and GASC present with metastatic disease. There is limited data about the surgery, radiotherapy and chemotherapy use in these rare and distinct pathologies. Data is limited to institutional case series and varies significantly. Additionally, population-based datasets such as SEER database lack chemotherapy data. Surgical resection for GSCC was reported to be utilized between 32% and 95% of cases in the literature, in comparison to 26% in the current study (1,16). Radiation therapy was used in approximately a third of the patients in the SEER database study and 48.1% in the current study, whereas no radiation was used in the study by Wakabayashi et al. (1,16). Use of adjuvant and neoadjuvant therapy ranges between 2% and 34% in the published literature. Current study points to significant differences in the practice patterns. In the absence of a standard therapy in these rare diseases and limited published data about use radiotherapy and systemic chemotherapy this study sheds light into current practice patterns in the US and provides a comprehensive analysis of the clinical outcomes.
Surgical resection was performed in about 77% of the GASC cases in two of the largest case series published in literature whereas 54.4% in the current study (14,18). Use of adjuvant therapy in published literature ranges between 15% and 46% (14,18). In the current study adjuvant chemotherapy and radiotherapy was administered in 11.9% and 11.9%, respectively. Use of radiotherapy, adjuvant and neoadjuvant chemotherapy in GSCC and GASC is not well defined in the literature and current study provides contemporary data about the trends of these modalities. It also provides stage specific treatment patterns. When the number of patients in the present study and nature of the database is considered it most likely represents current treatment patterns more accurately as opposed to institutional case series and case reports.
The clinical outcomes of GSCC and GASC individually and compared to GAC varies significantly in institutional case series and literature reviews. For example, 5-year OS of GSCC ranges between 13% and 32% in the literature and is 17.4% in this study (1,15). Median OS survival ranges between 7 and 8 months and is 8.9 months in this study (1,19). The 5-year OS of GASC ranges between 15.4% and 26.4% in the literature and is 14.7% in this study (18,20). The median OS ranges between 17 and 22 months, and is 9.9 months in the current study (14,18,21). These differences in outcomes may be due to sample size of the prior studies. When analyzed stage by stage GASC has the worst median survival for all stages compared to GSCC, and GAC in this study.
This study has limitations including the retrospective nature. The details of the chemotherapeutic regimens and the number of cycles of these therapies are not available in the data set. The extent of the surgical procedures and the dose of radiation therapy are not available. Nevertheless, this report includes the largest number of patients with GSCC and GASC, both exceedingly rare conditions, and sheds light into clinicopathological characteristics, current practice patterns and clinical outcomes in management of these clinically challenging entities.
Conclusions
GSCC and GASC are rare histologies and tend to present with more advanced stage disease. Even after controlling for all covariates, GSCC and GASC had significantly shorter overall survival compared to GAC.
Acknowledgements
Research reported in this publication was supported in part by the Winship Research Informatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The NCDB is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
References
- Dong C, Jiang M, Tan Y, et al. The clinicopathological features and prognostic factors of gastric squamous cell carcinoma. Medicine (Baltimore) 2016;95:e4720. [Crossref] [PubMed]
- Richman DM, Tirumani SH, Hornick JL, et al. Beyond gastric adenocarcinoma: Multimodality assessment of common and uncommon gastric neoplasms. Abdom Radiol (NY) 2017;42:124-40. [Crossref] [PubMed]
- Hwang SH, Lee JH, Kim K, et al. Primary squamous cell carcinoma of the stomach: A case report. Oncol Lett 2014;8:2122-4. [Crossref] [PubMed]
- Tokuhara K, Nakano T, Inoue K, et al. Primary squamous cell carcinoma in the gastric remnant. Surg Today 2012;42:666-9. [Crossref] [PubMed]
- Faria GR, Eloy C, Preto JR, et al. Primary gastric adenosquamous carcinoma in a Caucasian woman: a case report. J Med Case Rep 2010;4:351. [Crossref] [PubMed]
- Ajoodhea H, Zhang RC, Xu XW, et al. Fever as a first manifestation of advanced gastric adenosquamous carcinoma: a case report. World J Gastroenterol 2014;20:10193-201. [Crossref] [PubMed]
- Straus R, Heschel S, Fortmann DJ. Primary adenosquamous carcinoma of the stomach. A case report and review. Cancer 1969;24:985-95. [Crossref] [PubMed]
- Takita J, Kato H, Miyazaki T, et al. Primary squamous cell carcinoma of the stomach: a case report with immunohistochemical and molecular biologic studies. Hepatogastroenterology 2005;52:969-74. [PubMed]
- Boswell JT, Helwig EB. Squamous cell carcinoma and adenoacanthoma of the stomach. a clinicopathologic study. Cancer 1965;18:181-92. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma - 2nd English Edition. Gastric Cancer 1998;1:10-24. [Crossref] [PubMed]
- Parks RE. Squamous neoplasms of the stomach. Am J Roentgenol Radium Ther Nucl Med 1967;101:447-9. [Crossref] [PubMed]
- Shirahige A, Suzuki H, Oda I, et al. Fatal submucosal invasive gastric adenosquamous carcinoma detected at surveillance after gastric endoscopic submucosal dissection. World J Gastroenterol 2015;21:4385-90. [Crossref] [PubMed]
- Yoshida K, Manabe T, Tsunoda T, et al. Early gastric cancer of adenosquamous carcinoma type: report of a case and review of literature. Jpn J Clin Oncol 1996;26:252-7. [Crossref] [PubMed]
- Chen H, Shen C, Yin R, et al. Clinicopathological characteristics, diagnosis, treatment, and outcomes of primary gastric adenosquamous carcinoma. World J Surg Oncol 2015;13:136. [Crossref] [PubMed]
- Thomas RM, Sobin LH. Gastrointestinal cancer. Cancer 1995;75:154-70. [Crossref] [PubMed]
- Wakabayashi H, Matsutani T, Fujita I, et al. A rare case of primary squamous cell carcinoma of the stomach and a review of the 56 cases reported in Japan. J Gastric Cancer 2014;14:58-62. [Crossref] [PubMed]
- Bansal RK, Sharma P, Kaur R, et al. Primary gastric adenosquamous carcinoma in an Indian male. Indian J Pathol Microbiol 2013;56:416-8. [Crossref] [PubMed]
- Feng F, Zheng G, Qi J, et al. Clinicopathological features and prognosis of gastric adenosquamous carcinoma. Sci Rep 2017;7:4597. [Crossref] [PubMed]
- Meng Y, Zhang J, Wang H, et al. Poorer prognosis in patients with advanced gastric squamous cell carcinoma compared with adenocarcinoma of the stomach: Case report. Medicine (Baltimore) 2017;96:e9224. [Crossref] [PubMed]
- Chen YY, Li AF, Huang KH, et al. Adenosquamous carcinoma of the stomach and review of the literature. Pathol Oncol Res 2015;21:547-51. [Crossref] [PubMed]
- Saito S, Hosoya Y, Morishima K, et al. A clinicopathological and immunohistochemical study of gastric cancer with squamous cell carcinoma components: a clinically aggressive tumor. J Dig Dis 2012;13:407-13. [Crossref] [PubMed]