Detection of circulating tumor cells in cancers of biliary origin
Introduction
In In this era of personalized medicine there is a great need for individualizing therapy and better prognostic markers. Reproducible assays that can be used to determine the predictive value of promising biomarkers are fundamental to these efforts. Therapeutic and prognostic decisions are based today on clinical examination, imaging studies, on TNM staging system, molecular and receptor status and serum biomarkers. We rely largely on imaging assessment, however these are not ideal as it can take 2-3 months to see measurable response to therapy and in some circumstances patients do not have radiographically measurable disease (1). Furthermore with many biological therapies, radiographic responses are rare and sometimes even mild increase with central necrosis in the tumor is seen.
The principle that tumor cells overexpress epithelial cell adhesion molecule (EpCAM) has been exploited to detect these cells from peripheral blood using multiple techniques in various epithelial cancers over the past few years. Immunocytochemistry, reverse transcriptase-PCR, flow cytometry, the enzyme-linked immunosorbent spot (ELISPOT) assay, CellSearch and CellSpotter systems are some of the methods tested to detect the Circulating Tumor Cells(CTC) and determine their ability to predict treatment efficacy, progression free survival (PFS) and overall survival (OS) in patients with metastatic cancer (2-8). For most of these methods, lack of reproducibility has been the major limitation for widespread use.
The Circulating Tumor Cells (CTC) is a Clinical Laboratory Improvement Amendments (CLIA) validated assay that holds promise as it could serve as a surrogate marker in metastatic and primary epithelial cancers. CellSearch provided an immunomagnetic assay to capture and enumerate CTCs by targeting the overexpressed EpCAM using antibodies against CD45-, EpCAM+, DAPI+, CK (cytokeratin) 8, 18 and/or 19+. The assay was validated by achieving high sensitivity, specificity, positive and negative predictive values (98%, 100%, 96.5% and 100% respectively) (9). It is an automated, standardized, reproducible and FDA approved assay for use in metastatic breast (1,10), colon (11,12) and prostate cancer (13,14). CTC detection has been undertaken using this assay in bladder cancer (15-17), melanoma (18), lung (19) in addition to other solid tumors. CTC detection using Cellsearch has also been evaluated in small studies to monitor chemotherapy outcomes in hepatocellular and pancreatic cancer (20,21).
Cholangiocarcinoma is a rare type of cancer with rising incidence worldwide (22) and carries a very poor prognosis. Cancers of the bile duct include tumors arising in the intra and extra hepatic bile ducts (called cholangiocarcinoma) as well as cancer of the gall bladder. Most patients have advanced disease at presentation and survival is often short. Therapy for these patients is intended to be palliative and quality of life is often poor, hence early knowledge of the likelihood of benefit from different therapies could be very useful to guide management. No studies have yet been done to examine the detection of CTCs in cholangiocarcinoma and gallbladder cancer using immunomagnetic assays thus the cut off for a positive CTC value has not yet been defined. Since EpCAM is overexpressed in gallbladder cancer and cholangiocarcinoma (63-100 and 81-90% respectively) (23) we hypothesized that CTCs can be detected and might correlate with stage of disease.
Methods
Patients
With IRB approval, sixteen patients from Roswell Park Cancer Institute with cholangiocarcinoma or gallbladder cancer that had at least one CTC measurement were included. This included all patients with histological diagnosis of cholangiocarcinoma or gallbladder cancer which was the only inclusion criteria. Samples were collected and analyzed using the CellSearch assay. Clinical follow up and sample collection occurred between June 2008 and May 2011.
To evaluate patients survival and its correlation with CTC status, patients were divided into two groups, group one (G1) were patients with negative or 1CTC/7.5 mL and group two (G2) were patients with 2 or more CTC/ 7.5 mL. Overall survival was defined as the time of follow up between the first CTC detected and the survival (censored at May 2011). Progression free survival was defined as the time of follow up between the first CTC and the first progression on CT scans according to WHO criteria.
CTC assay
The circulating tumor cells (CTC) were detected using the CellSearch assay by Veridex LLC, Raritan, NJ (24,25). The CellSearch assay enumerates only CTC that express EpCAM and cytokeratins (CK) 8, 18, and 19. The CellSearch epithelial cell kit (Veridex LLC) contains an anti-EpCAM ferrofluid capture reagent and immunofluorescent reagents. The anti-EpCAMferrofluid reagent consists of nanoparticles with a magnetic core surrounded by a polymeric layer coated with monoclonal antibodies targeting the EpCAM antigen expressed by CTCs allowing their selective capture. After immunomagnetic capture of CTCs and enrichment, fluorescent reagents were added for identification and enumeration of CTCs. The fluorescent reagents included: a mixture of two phycoerythrin (PE) conjugated anti-CK monoclonal antibodies that bind to CTCs intracellular cytokeratins 8, 18, 19 and nucleic acid dye 4', 6-diamidino-2-phenylindole (DAPI) to fluorescently label the cells nuclei and anti-CD45-Allophycocyanin (APC) conjugated monoclonal antibodies specific for leukocytes.
Peripheral blood (7.5 mL) was collected into CellSave Preservative Tubes (Veridex LLC, Raritan, NJ), which are evacuated blood draw tubes containing EDTA as anticoagulant and a cellular preservative. Blood samples were maintained at room temperature for different time intervals and processed within a maximum of 96 h after blood drawing (2,26). One representative sample result is shown in Figure 1.
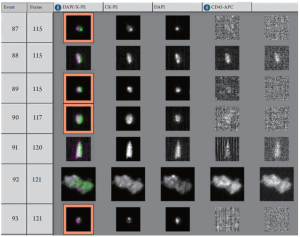
A positive CTC was defined at two cells per 7.5 mL of blood based on the expected frequency of EpCAM detection in patients with biliary cancer.
No spiking experiments were done in biliary cancer cells. The CellSearch positive control kit is used to maintain quality control for reagents, instruments and operator techniques and is performed for each patient's sample. The control kit includes 24 single use control bottles containing two populations of a fixed breast cancer cell line at high and low concentrations. Different fluorescent dyes specific to each populations permit simultaneous enumeration of high and low controls in one assay. Each of the high and low CTC control values obtained has to fit within two standard deviation of the relevant reference range mean.
Results
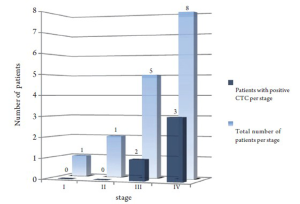
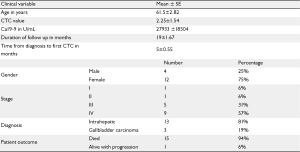
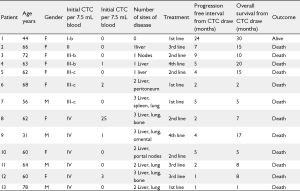
Patients' baseline characteristics are shown in Table 1. Sixteen patients were identified and included; mean age at the time of diagnosis was 62 (31-78) years. Twelve patients (75%) were females, 13 (81%) had intrahepatic cholangiocarcinoma, three patients had gallbladder cancer. Stages at assessment of CTC were: Stage I (n=1), stage II (n=1), stage III (n=5) and stage IV (n=9). Fifteen patients (94%) died during the time of follow up. Mean followup time was twenty months. Patients' therapies and outcome details are shown in Tables 2 and 3.
Full table
Full table
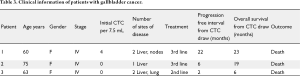
Full table
Using 2 CTC/7.5 mL as a cutoff; 3/13 patients with cholangiocarcinoma and 1/3 with gallbladder cancer were positive. All patients with positive CTC had stage III or IV when the assay was conducted as shown in figure 2 .
When studying patients' outcome and the correlation with CTC value at 12 months of follow up from time CTC was drawn; 1/4(25%) of patients with positive CTC and 6/12(50%) of patients with negative CTC remained alive which was not statistically significant (P=0.392) using Fisher exact test. Overall survival between the two groups was not statistically significant using Kaplan Meier curve (P=0.796). However this is a pilot study that was not designed to compare overall survivals between patients with negative and positive CTC.
Discussion
The CellSearch is the first automated, standardized, reproducible and FDA approved test for use in advanced metastatic breast (1,10), colon (11,12) and prostate cancer (13,14). Patients with positive CTC at baseline were predicted to have shorter progression free survival (2.7 vs. 7.0 months, 4.5 vs. 7.9 months, 4.2 vs. 5.8 months for breast, colorectal and prostate cancers, respectively) and overall survival (10.9 vs. 21.9 months, 9.4 vs. 18.5 months and 11.5 vs. 21.7 months for breast, colorectal and prostate cancers, respectively) when compared with patients with negative CTC. The same assay has been investigated in other solid tumors including melanoma (18), urothelial cancer (15-17), pancreatic (20,21) and lung cancer (19).
The concept of detecting CTC in biliary cancer was previously described but using a completely different technique based on carcinoembryonic antigen reverse transcriptase polymerase chain reaction (CEA RT-PCR) but none described CTC detection with an immunomagnetic assay (27). The strengths of this study are the use of a validated CLIA approved CellSearch system from Veridex to detect and enumerate the CTCs in the peripheral blood and the first report of detecting these cells in patients with gallbladder cancer and cholangiocarcinoma due to high frequency of EpCAM over expression (63-100% and 81-90% respectively according to different authors) (23,28-30) where it is described to be overexpressed in breast cancer in 81-100% in most of the subtypes with few exceptions (29). Cytokeratins 7, 8, 18, 19 and 20 were immunohistochemically examined in intrahepatic cholangiocarcinoma tissue samples and were found to be expressed in 97, 97, 77 and 71% of immunohistochemistry respectively. Relatively similar results were found in samples from gallbladder cancer (31).
Similar pilot studies have been used to help define a positive value which differs for each tumor type. Selection of the cut off value for positive CTC is based on EpCAM expression, detection rates and prospective validation of its prognostic significance by statistical analysis. Positive CTC values are defined differently in different cancers (3CTC/7.5 mL for colorectal cancer, 5CTC/7.5 mL for breast and prostate cancers) (8) which is related to the variation (32) or loss in the expression of EpCAM (33). The cut off for CTC has not yet been defined in biliary cancer and in this study we propose to set it at 2 CTC/7.5 mL. The measurement of one CTC/7.5 mL can be incidentally seen from healthy subjects or patients with benign breast and colon conditions and no studies have shown predictive value of one CTC/7.5 mL with outcomes (8). The same cut off (2 CTCs) was used for bladder cancer where EpCAM detection is about 35% (8,25).
The reasons why there is variability in detection rates compared to immunohistochemical expression of EpCAM are not entirely clear. The lack of the ability to detect CTCs in a higher percentage of patients with metastatic cancer may be due to the epithelial-mesenchymal transition (EMT), less expression of epithelial surface antigens (1) and there less EpCAM detection by CellSearch technology in advanced cancer (34). A recent study using the CellSearch system has shown that normal-like breast cancer subtype cell line with features of EMT has EpCAM levels that are too low to allow capture using their antibodies which raises the importance of developing alternative CTC markers in such specific circumstances (35). In colorectal cancer, in spite of EpCAM overexpression in almost 100% of cells on immunochemistry, detection can be as low as 25% where the cut off is set for 3 CTC/7.5 mL using CellSearch assay (8,26,29,36). Our results support that the same concept can be applied to biliary cancer as 25% of the patients had two or more CTCs/7.5 mL, when tumor EpCAM Expression ranges from 63-100% in cholangiocarcinoma and 81-90% in gallbladder cancer. Some drawbacks of this study are the lack of control healthy donors to evaluate false positive results. However it is expected to be very low based on similar examples of published breast and colon cancer studies. As this was intended as a pilot study, there was also some heterogeneity in advanced cancer patients as some were not treatment naïve and some opted for supportive care during the followup period. As changes in CTCs in circulation can be a function of disease burden and response to therapy, this limits a true assessment of the frequency of CTC detection at diagnosis in this illness. However this may not be a major drawback in the study design since positive CTCs correlated with poor outcome even at different intervals of the follow up in patients with metastatic breast cancer (8).
Another drawback would be the source of the CTC in those patients, all patients underwent surgical or radiology guided biopsies of their initial tumors including all the liver masses outlined in Tables 1 and 3 which were all positive for their respective diagnosis of either gallbladder cancer or cholangiocarcinoma. However, lung or bone involvements of disease were not required to be biopsy proven per standards of care as patients' radiological staging was consistent with metastases from their pathology proven initial cholangiocarcinoma or gallbladder cancer without any evidence of the presence of other primary tumors in those patients.
As a clinical observation, two patients had serial CTC values in their disease course. In both patients CTC values correlated with the burden of their disease where decreased CTC were seen after treatment and this was associated in clinical and radiological improvement. This observation merits further validation as both baseline and early change in CTCs may prove to be useful to guide therapeutic decisions and to predict clinical outcomes.
Conclusions
This is the first report to show a clinical observation of detectable CTCs in patients with cancers of biliary origin. In this pilot study using a cutoff of 2CTCs/7.5 mL, 25% of patients with biliary cancer had detectable CTCs. Our results suggest that positive as well as negative CTC results may have prognostic value in predicting outcomes but need prospective validation. Our group is currently conducting a prospective study to determine the value of baseline and change in CTCs during chemotherapy. This trial may help define the optimal CTC cutoff in predicting clinical outcomes in advanced biliary cancer patients.
Funding
Dr. Iyer is supported by a grant from the American Cancer Society (MSRG -08-096-01-CCE). This research was supported, in part, by the National Cancer Institute (NCI) Support Grant to the Roswell Park Cancer Institute [P30 CA016056].
Footnote
No potential conflict of interest.
References
- Budd GT, Cristofanilli M, Ellis MJ, et al. Circulating tumor cells versus imaging--predicting overall survival in metastatic breast cancer. Clin Cancer Res 2006;12:6403-6409. [PubMed]
- Alunni-Fabbroni M, Sandri MT. Circulating tumour cells in clinical practice: Methods of detection and possible characterization. Methods 2010;50:289-297. [PubMed]
- Shariat SF, Roudier MP, Wilcox GE, et al. Comparison of immunohistochemistry with reverse transcription-PCR for the detection of micrometastatic prostate cancer in lymph nodes. Cancer Res 2003;63:4662-4670. [PubMed]
- Pantel K, Schlimok G, Angstwurm M, et al. Methodological analysis of immunocytochemical screening for disseminated epithelial tumor cells in bone marrow. J Hematother 1994;3:165-173. [PubMed]
- Gomella LG, Raj GV, Moreno JG. Reverse transcriptase polymerase chain reaction for prostate specific antigen in the management of prostate cancer. J Urol 1997;158:326-337. [PubMed]
- de la Taille A, Muscatelli B, Colombel M, et al. Prog Urol 1998;8:1058-1064. [In vitro detection of prostate cancer circulating cells by immunocytochemistry, flow cytometry and RT-PCR PSA]. [PubMed]
- Alix-Panabières C, Vendrell JP, Pellé O, et al. Detection and characterization of putative metastatic precursor cells in cancer patients. Clin Chem 2007;53:537-539. [PubMed]
- Miller MC, Doyle GV, Terstappen LW. Terstappen, Significance of Circulating Tumor Cells Detected by the CellSearch System in Patients with Metastatic Breast Colorectal and Prostate Cancer. J Oncol 2010;2010:617421.Epub 2009 Dec 9.
- Gazzaniga P, Naso G, Gradilone A, et al. Chemosensitivity profile assay of circulating cancer cells: prognostic and predictive value in epithelial tumors. Int J Cancer 2010;126:2437-2447. [PubMed]
- Cristofanilli M, Broglio KR, Guarneri V, et al. Circulating tumor cells in metastatic breast cancer: biologic staging beyond tumor burden. Clin Breast Cancer 2007;7:471-479. [PubMed]
- Cohen SJ, Punt CJ, Iannotti N, et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J Clin Oncol 2008;26:3213-3221. [PubMed]
- Locker GY, Hamilton S, Harris J, et al. ASCO 2006 update of recommendations for the use of tumor markers in gastrointestinal cancer. J Clin Oncol 2006;24:5313-5327. [PubMed]
- de Bono JS, Scher HI, Montgomery RB, et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin Cancer Res 2008;14:6302-6309. [PubMed]
- Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 1999;17:3461-3467. [PubMed]
- Rink M, Chun FK, Minner S, et al. Detection of circulating tumour cells in peripheral blood of patients with advanced non-metastatic bladder cancer. BJU Int 2011;107:1668-1675. [PubMed]
- Okegawa T, Hayashi K, Hara H, Nutahara K, Higashihara E. Immunomagnetic quantification of circulating tumor cells in patients with urothelial cancer. Int J Urol 2010;17:254-258. [PubMed]
- Guzzo TJ, McNeil BK, Bivalacqua TJ, Elliott DJ, Sokoll LJ, Schoenberg MP. The presence of circulating tumor cells does not predict extravesical disease in bladder cancer patients prior to radical cystectomy. Urol Oncol 2012;30:44-48. [PubMed]
- Steen S, Nemunaitis J, Fisher T, Kuhn J. Circulating tumor cells in melanoma: a review of the literature and description of a novel technique. Proc 2008;21:127-132. Bayl Univ Med Cent. [PubMed]
- Hou JM, Greystoke A, Lancashire L, et al. Evaluation of circulating tumor cells and serological cell death biomarkers in small cell lung cancer patients undergoing chemotherapy. Am J Pathol 2009;175:808-816. [PubMed]
- Vizio B, Novarino A, Giacobino A, et al. Pilot study to relate clinical outcome in pancreatic carcinoma and angiogenic plasma factors/circulating mature/progenitor endothelial cells: Preliminary results. Cancer Sci 2010;101:2448-2454. [PubMed]
- Zhou J, Hu L, Yu Z, et al. Marker expression in circulating cancer cells of pancreatic cancer patients. J Surg Res 2011;171:631-636. [PubMed]
- Kamphues C, Seehofer D, Eisele RM, et al. Recurrent intrahepatic cholangiocarcinoma: single-center experience using repeated hepatectomy and radiofrequency ablation. J Hepatobiliary Pancreat Sci 2010;17:509-515. [PubMed]
- Kawashima R, Abei M, Fukuda K, et al. EpCAM- and EGFR-targeted selective gene therapy for biliary cancers using Z33-fiber-modified adenovirus. Int J Cancer 2011;129:1244-1253. [PubMed]
- Naoe M, Ogawa Y, Takeshita K, Iwamoto S, Miyazaki A. Use of the CellSearch Circulating Tumor Cell Test for monitoring urothelial cancer: two case reports of metastatic urothelial cancer. South Med J 2008;101:439-441. [PubMed]
- Naoe M, Ogawa Y, Morita J, et al. Detection of circulating urothelial cancer cells in the blood using the CellSearch System. Cancer 2007;109:1439-1445. [PubMed]
- Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res 2004;10:6897-6904. [PubMed]
- Uchikura K, Takao S, Nakajo A, et al. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann Surg Oncol 2002;9:364-370. [PubMed]
- de Boer CJ, van Krieken JH, Janssen-van Rhijn CM, Litvinov SV. Expression of Ep-CAM in normal, regenerating, metaplastic, and neoplastic liver. J Pathol 1999;188:201-206. [PubMed]
- Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol 2004;35:122-128. [PubMed]
- Varga M, Obrist P, Schneeberger S, et al. Overexpression of epithelial cell adhesion molecule antigen in gallbladder carcinoma is an independent marker for poor survival. Clin Cancer Res 2004;10:3131-3136. [PubMed]
- Shimonishi T, Miyazaki K, Nakanuma Y. Cytokeratin profile relates to histological subtypes and intrahepatic location of intrahepatic cholangiocarcinoma and primary sites of metastatic adenocarcinoma of liver. Histopathology 2000;37:55-63. [PubMed]
- Witzig TE, Bossy B, Kimlinger T, et al. Detection of circulating cytokeratin-positive cells in the blood of breast cancer patients using immunomagnetic enrichment and digital microscopy. Clin Cancer Res 2002;8:1085-1091. [PubMed]
- Smirnov DA, Zweitzig DR, Foulk BW, et al. Global gene expression profiling of circulating tumor cells. Cancer Res 2005;65:4993-4997. [PubMed]
- Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer 2007;96:1013-1019. [PubMed]
- Sieuwerts AM, Kraan J, Bolt J, et al. Anti-epithelial cell adhesion molecule antibodies and the detection of circulating normal-like breast tumor cells. J Natl Cancer Inst 2009;101:61-66. [PubMed]
- Denda-Nagai K, Irimura T. MUC1 in carcinoma-host interactions. Glycoconj J 2000;17:649-658. [PubMed]