
The impact of BMI extremes on disease-free survival and overall survival following cytoreductive surgery and hyperthermic intraperitoneal chemotherapy
Introduction
The excess of body fat content, as measured by increased body mass index (BMI), has been associated with an increased risk of several cancer occurrences. The International Agency for Research on Cancer (IARC) confirmed the correlation between obesity and many malignancies such as esophageal, gastric cardia, hepatobiliary, colorectal, gynecologic, and postmenopausal breast cancers (1). This correlation has been reputable through several meta-analyses and reviews (2,3). Moreover, the biologic basis of causality was explained by systemic and microenvironmental immune responses, insulin-like growth factors, and steroid hormones pathways (4-6), further clarifying and contribution of obesity in the increasing incidence of cancer.
However, studying the impact of obesity on survival after the diagnosis of cancer in the recent years revealed rather surprising results. Obese patients with colorectal cancer, B-cell lymphomas and renal cell carcinomas demonstrated lower rates of mortality and improved survival compared to their peers of normal weight (7-10). This “obesity paradox” that refers to the improved survival in obese patients, in contrary to the common or expected belief, is an evident phenomenon in other disciplines of healthcare. Obese end-stage renal disease (ESRD) patients on renal replacement therapy outlive their normal-weight peers and outlive their hemodialysis accesses (11). Similarly, obese patients with coronary disease and type II diabetes tend to have a longer survival than those with normal BMI (12-14). On the other hand, the role of BMI, as a routinely reported physiologic parameter, in cancer recurrence has been an area of debate. Visceral adiposity, as measured by visceral fat area on computed tomography, has shown to correlate better with disease recurrence in rectal cancer compared to BMI which produces variable observations and less accurate recurrence prediction (15). In ovarian cancer, increased BMI was associated with a higher likelihood to have the disease limited to the ovaries, but clearly associated with a higher risk of disease recurrence and shorter overall survival (OS) when the disease is spread outside the adnexa (i.e., stages III–IV) (16).
In the current practice, peritoneal carcinomatosis (PC) is approached with a potentially curative intent by offering cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS and HIPEC) to a selected group of patients, in whom the most common origin of PC is gynecologic and colorectal. The relation between BMI and disease-free survival (DFS) and OS has not been explicitly studied in CRS and HIPEC despite the consensus on the importance of the nutritional status on long-term oncologic outcomes. Herein, we aim to study the immediate outcomes, as well as DFS/OS of patient from different BMI strata after CRS and HIPEC.
Methods
This is a retrospective analysis of patients who underwent CRS and HIPEC between 2007–2017 at a single institution for diagnoses of PC from colorectal (including appendiceal) adenocarcinoma, ovarian epithelial cancer, and pseudomyxoma peritonei (PMP). All patients had their demographic and perioperative characteristics collected and stored in a prospectively maintained database in accordance with an IRB-approved protocol at our institution (EEH 00003456). Comorbidities were reported per the Charlson comorbidity index (17). Peritoneal carcinomatosis index (PCI) was calculated intraoperatively as described by Dr. Sugarbaker (18). Grading of postoperative complications was done in accordance with the Clavien-Dindo classification system (19).
Patients were followed postoperatively with serial computed tomographies every 3 months for 2 years along with tumor markers, based on which disease recurrence was detected and determined. We divided the patients according to their BMI strata into underweight (BMI <18.50 kg/m2), normal weight (BMI 18.50–24.99 kg/m2), overweight (BMI 25.00–29.99 kg/m2), obese (BMI 30.00–34.99 kg/m2), and morbidly obese (BMI ≥35.00 kg/m2) groups. Primary outcomes of the study were DFS and OS. Secondary goal was the comparison of immediate surgical outcomes between the different BMI groups.
We used the standard inferential tests to compare the characteristics between the groups including Chi-square, Kruskal-Wallis, and ANOVA. Kaplan-Meier method was utilized to draw the DFS and OS plots and compare medians. Cox-regression analysis was applied to determine the independent predictors of DFS and OS. Joinpoint regression was used to determine the optimal BMI for DFS and OS with tumor histology as the by-variable. Significance was set at <0.05 throughout the analysis.
Results
A total of 126 patients who underwent CRS and HIPEC were included. Mean age was 59.31±1.57 years, and 54 (42.9%) were males. The breakdown of the primary histology was appendiceal (n=22; 17.5%), colorectal (n=38; 30.2%), ovarian (n=19; 15.1%), and PMP (n=47; 37.3%). Appendiceal and colorectal adenocarcinoma were bundled during the analysis of survival. The median follow-up was 19 months. Two patients had repeat CRS and HIPEC for disease recurrence and one patient had two reoperations during the study period. Each patient was included in the analysis as a single entry. The operative outcomes of the re-operations were not analyzed given the inherent bias in case complexity and survival expectancy. Patients were recorded to have a recurrence at time of reoperation and followed to measure the OS.
Mean PCI was 14.06±9.33, and 90.5% of patients underwent macroscopic cytoreduction. Eleven point one percent of patients had major morbidities (grade III–IV) and two postoperative mortalities were reported. Demographic and perioperative characteristics of our patient population are summarized in Table 1.

Full table
When patients were divided per their BMI strata as described, no significant difference was noted between the groups in regards to demographics, operative details, and postoperative complications despite a trend toward a higher PCI and lower rates of complete cytoreduction in the underweight group. Comparative results of the BMI groups are shown in Table 2.
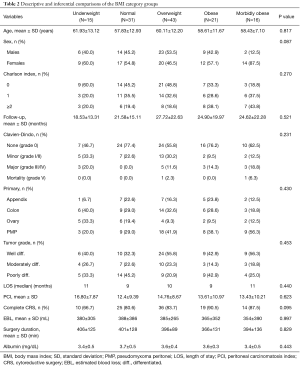
Full table
Kaplan-Meier survival plots demonstrated that the underweight, normal weight, and morbidly obese groups had significantly shorter medians of DFS and OS compared to the overweight, obese, and morbidly-obese groups. No difference was noted in DFS between the overweight, obese, and morbidly-obese groups. Morbidly-obese patients had shorter OS compared to overweight and obese groups (48.00±23.20 vs. 66.00±12.66 and 68.00±27.74 months, respectively; P=0.011). Kaplan-Meier curves for DFS and OS are demonstrated in Figure 1. Univariate and multivariate Cox regression analyses were applied to determine the independent predictors of DFS and OS in our patient population. Our results suggest that tumor grade and incomplete cytoreduction were independent predictors of recurrence and long-term survival. In regards to BMI groups, being underweight carried a poor prognosis if diagnosed with PC for DFS and OS, whereas being morbidly-obese was a poor prognosticator for OS only. There was no difference in DFS or OS in the overweight and obese groups. Results of the Cox regression are shown in Table 3.
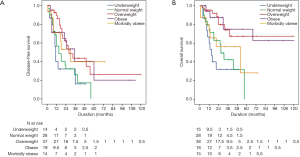
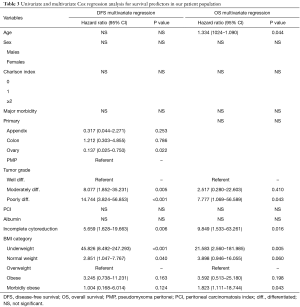
Full table
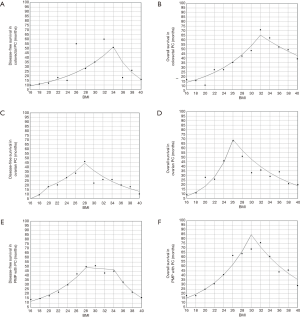
Finally, joinpoint regression was applied to study the trend of DFS and OS across the BMI spectrum. Patients were studied per their primary histology. Our results indicate that optimum BMI for DFS and OS in colorectal PC were 34 and 32 kg/m2, respectively; 28 and 26 kg/m2 in ovarian PC, respectively; and 28 and 30 kg/m2 for PMP, respectively. Results of the Joinpoint regression analysis are demonstrated in Figure 2.
Discussion
It is currently projected that two-thirds of adults in the United States are considered overweight or obese (20), with a striking expansion of the morbid obesity group, faster than the overall increase in the obesity prevalence itself (21,22). This alarming fact indicates that facing obesity and morbid obesity will become a routine challenge in every surgical discipline in the years to come.
As the application of CRS and HIPEC for peritoneal surface malignancies (PSM) is increasing worldwide, it becomes imperative to understand the influence of obesity on the short- and long-term outcomes of CRS and HIPEC. Furthermore, we noted that the impact of high BMI on the oncologic outcome has been investigated more thoroughly than that of low BMI. Therefore, we aimed in this work to study how BMI, as a spectrum, would influence the patients’ survival as it trends towards either extreme.
Our results suggest a variability in recurrence and survival outcomes across the BMI range based on the histology of the primary tumor. Overweight patients with PC from ovarian origin tend to have an optimal DFS and OS which worsen as BMI moves toward either BMI extreme. Albeit HIPEC did not take part in the surgical management in their study, Tran et al. (23) demonstrated in their study on 104 patients that optimal cytoreduction in disseminated ovarian cancer is not affected by BMI. Rather, it is the long-term survival that is inversely proportional to the increasing BMI strata.
Upon further assessment of our patient subgroups, we redemonstrate the protective impact of obesity in PSM from appendiceal and colorectal origins, as the optimum of BMI for DFS and OS were 34 and 32, respectively. Votanopoulos et al. (24) reported similar conclusions of comparable short-term outcomes, yet improved survival in obese patients with PSM of lower gastrointestinal origin after CRS and HIPEC. Additionally, in our study we report that survival in PMP follows a similar pattern in regards to BMI stratification, with borderline-obesity being the optimum for long-term survival.
In regards to our secondary outcomes, our data are in agreement with several other institutional and national registry analyses that obesity does not impose a significant increase in immediate complications following CRS and HIPEC (24-26). What we highlight in our study is that extremely low BMI, a widely understudied risk factor, was associated with worse DFS and OS in all histology subgroups. It is worth mentioning that underweight patients had a trend toward a higher PCI mean and lower rates of CC0/1, indicating a wider spread of their disease. Nonetheless, the BMI stratum of underweight was an independent predictor of poorer DFS and OS compared to the normal weight patients in our regression model. Of note, extremely low BMI was not necessarily associated with significantly lower levels of preoperative albumin. Increased tumor burden and cachexia would be expected etiologies of low BMI, higher PCI, thus lower rates of CC0/1 in the underweight group. However, that was not evident in our patient population.
On the other extreme, albeit a comparable DFS in morbidly obese patients their weight stratum reflected a significantly shorter OS. This finding might suggest that morbid obesity imposes a risk of non-cancer related death in the years that follow CRS and HIPEC.
The shortcomings of this study revolve around the retrospective nature of the analysis and the long-span of inclusion. They both contribute to data loss, inconsistency in data collection, and heterogeneity within the groups in the study. The approaches and techniques of CRS and HIPEC were standardized during the period of the study leading to improved outcomes, in addition to the relatively long learning curve this complex procedure requires till proficiency (27,28). However, we attempted to compensate for these inherent limitations by excluding patients with missed data points, as well as by following the proper statistical methods to adjust for potential confounders as described in the methods.
Conclusions
Our analysis of BMI, as a spectrum, concludes that weight strata affect OS and DFS per the primary histology. Ovarian PC demonstrates earlier recurrence and shorter survival, whereas colorectal PC demonstrates the “obesity paradox” as patients move into the realm of obesity. BMI extremes, low or high, generally carry a poor prognosis for OS and DFS in PSM patients undergoing CRS and HIPEC compared to their normal-weight peers.
Acknowledgements
None.
Footnote
Conflicts of Interest: This work has been presented at the SSO 13th International Symposium of Regional Cancer Therapies 2018 in Jacksonville, FL.
Ethical Statement: Our study is conducted under an approved IRB protocol (EEH 00003456) and patients’ informed consent, and is enrolled in the National Clinical Trials registry NCT02082886.
References
- Lauby-Secretan B, Scoccianti C, Loomis D, et al. Body Fatness and Cancer--Viewpoint of the IARC Working Group. N Engl J Med 2016;375:794-8. [Crossref] [PubMed]
- Kyrgiou M, Kalliala I, Markozannes G, et al. Adiposity and cancer at major anatomical sites: umbrella review of the literature. BMJ 2017;356:j477. [Crossref] [PubMed]
- Park Y, Colditz GA. Fresh evidence links adiposity with multiple cancers. BMJ 2017;356:j908. [Crossref] [PubMed]
- Iyengar NM, Gucalp A, Dannenberg AJ, et al. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J Clin Oncol 2016;34:4270-6. [Crossref] [PubMed]
- Renehan AG. Hormones, Growth Factors, and Tumor Growth. Oxford, UK: Oxford University Press, 2007.
- Renehan AG, Harvie M, Howell A. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and breast cancer risk: eight years on. Endocr Relat Cancer 2006;13:273-8. [Crossref] [PubMed]
- Schlesinger S, Siegert S, Koch M, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control 2014;25:1407-18. [Crossref] [PubMed]
- Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 2013;132:625-34. [Crossref] [PubMed]
- Weiss L, Melchardt T, Habringer S, et al. Increased body mass index is associated with improved overall survival in diffuse large B-cell lymphoma. Ann Oncol 2014;25:171-6. [Crossref] [PubMed]
- Carson KR, Bartlett NL, McDonald JR, et al. Increased body mass index is associated with improved survival in United States veterans with diffuse large B-cell lymphoma. J Clin Oncol 2012;30:3217-22. [Crossref] [PubMed]
- Schmidt D, Salahudeen A. The obesity-survival paradox in hemodialysis patients: why do overweight hemodialysis patients live longer? Nutr Clin Pract 2007;22:11-5. [Crossref] [PubMed]
- Doehner W, Erdmann E, Cairns R, et al. Inverse relation of body weight and weight change with mortality and morbidity in patients with type 2 diabetes and cardiovascular co-morbidity: an analysis of the PROactive study population. Int J Cardiol 2012;162:20-6. [Crossref] [PubMed]
- Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med 2007;120:863-70. [Crossref] [PubMed]
- Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohort studies. Lancet 2006;368:666-78. [Crossref] [PubMed]
- Ballian N, Lubner MG, Munoz A, et al. Visceral obesity is associated with outcomes of total mesorectal excision for rectal adenocarcinoma. J Surg Oncol 2012;105:365-70. [Crossref] [PubMed]
- Pavelka JC, Brown RS, Karlan BY, et al. Effect of obesity on survival in epithelial ovarian cancer. Cancer 2006;107:1520-4. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 2009;250:187-96. [Crossref] [PubMed]
- Flegal KM, Carroll MD, Ogden CL, et al. Prevalence and trends in obesity among US adults, 1999-2000. JAMA 2002;288:1723-7. [Crossref] [PubMed]
- Howard NJ, Taylor AW, Gill TK, et al. Severe obesity: Investigating the socio-demographics within the extremes of body mass index. Obes Res Clin Pract 2008;2:I-II. [Crossref] [PubMed]
- Sturm R. Increases in morbid obesity in the USA: 2000-2005. Public Health 2007;121:492-6. [Crossref] [PubMed]
- Tran AQ, Cohen JG, Li AJ. Impact of obesity on secondary cytoreductive surgery and overall survival in women with recurrent ovarian cancer. Gynecol Oncol 2015;138:263-6. [Crossref] [PubMed]
- Votanopoulos KI, Swords DS, Swett KR, et al. Obesity and peritoneal surface disease: outcomes after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for appendiceal and colon primary tumors. Ann Surg Oncol 2013;20:3899-904. [Crossref] [PubMed]
- Neuwirth MG, Bartlett EK, Roses RE, et al. Obesity is not associated with increased morbidity in patients undergoing cytoreductive surgery with intraperitoneal chemotherapy. J Surg Oncol 2016;114:619-24. [Crossref] [PubMed]
- Polanco PM, Sanchez AI, Ramalingam L, et al. Does obesity affect outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for disseminated mucinous appendiceal neoplasms? Ann Surg Oncol 2014;21:3963-9. [Crossref] [PubMed]
- Chua TC, Liauw W, Saxena A, et al. Evolution of locoregional treatment for peritoneal carcinomatosis: single-center experience of 308 procedures of cytoreductive surgery and perioperative intraperitoneal chemotherapy. Am J Surg 2011;201:149-56. [Crossref] [PubMed]
- Votanopoulos KI, Newman NA, Russell G, et al. Outcomes of Cytoreductive Surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in patients older than 70 years; survival benefit at considerable morbidity and mortality. Ann Surg Oncol 2013;20:3497-503. [Crossref] [PubMed]


