Inflammatory response and optimalisation of perioperative fluid administration during hyperthermic intraoperative intraperitoneal chemotherapy surgery
Introduction
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (HIPEC) is a treatment option for selected patients with peritoneal carcinomatosis. Unfortunately, this procedure has a high incidence of postoperative complications (1,2). HIPEC only little contributes to the morbidity and mortality which are more dependent on the surgery itself (3). Inadequate intraoperative volume therapy is a known risk factor for complications. Previous studies have documented haemodynamic changes associated with HIPEC, but the optimal strategy for patient management during administration of intraperitoneal chemotherapy is unclear (4-6). Critical evaluation of fluid status is challenging in patients undergoing HIPEC due to preoperative fasting, epidural analgesia, accumulation of subcutaneous fluid, bleeding and insensible fluid loss. It is common practice to compensate perioperatively for the fasting status with 5–10 mL per kg body weight, and for the effect of the epidural analgesia with a 500-mL fluid bolus. Furthermore, during the operation fluid at 10 mL/kg/h is usually administered. This practice is not patient tailored. Urine output is an unreliable measure of fluid status during HIPEC due to administration of various medication, e.g., opiates, inotropic support, and diuretics (7). As a consequence, most patients undergoing HIPEC receive at least 7 L of intravenous fluids during the procedure (8).
Continuous monitoring of haemodynamic parameters improves estimation of the fluid balance (9). Goal-directed fluid management may result in a more restricted fluid administration (10), fewer complications (11), a faster postoperative recovery (12) and a cost reduction by approximately 25% (13). Whereas, more liberal fluid administration leads to increased fluid accumulation as part of the perioperative inflammatory response, which may compromise the microcirculation. For instance, alveolar edema delays extubation and prolongs the postoperative ventilation period, hospital stay and increases hospital costs (14). A recent clinical trial however did not find these advantages of restrictive fluid management (15).
Multiple methods have been developed to monitor haemodynamics of which pulmonary artery catheter thermodilution is the gold standard. However, this invasive technique may be associated with various serious complications, e.g., arrhythmia, pneumothorax, infections, and pulmonary artery perforation (16,17), which have an incidence of approximately 5% (18). Moreover, placement of pulmonary artery catheter has never been shown to be of benefit. Non-invasive techniques have been developed that are considered to be safer. One of these techniques is FloTrac/Vigileo (Edwards Lifesciences, Irvine, California, USA), which uses an arterial line to measure the area under the curve of the blood pressure. Together with the patient’s characteristics, e.g., age, gender, height and weight, the stroke volume (SV), cardiac output (CO) and SV variation is calculated (19,20).
Major surgery induces a systemic inflammatory response by generating a multitude of inflammatory mediators. The severity of this response may impact on clinical outcome. For example, postoperative interleukin-6 (IL-6) levels correlate with cardiac complications in patients undergoing major abdominal surgery (21).
We conducted an observational randomised pilot to test the hypothesis that in patients undergoing HIPEC a 30% reduction of fluid administration could be accomplished with the use of the FloTrac/Vigileo monitoring system. Secondly, we measured circulating cytokines and evaluated the possible relation of these changes of inflammatory response with the non-invasive monitored fluid management by FloTrac/Vigileo.
Methods
Study design
The aim of the present randomised trial was to evaluate if patients undergoing a HIPEC procedure received a more optimal fluid administration if FloTrac/Vigileo monitoring was used compared with patients receiving standard care. Furthermore, we evaluated the occurrence and severity of the inflammatory response within 48 hours after the HIPEC procedure. Levels of various inflammatory mediators, such as interleukins, neutrophil degranulation products, complement, and C-reactive protein (CRP) were measured at seven time points during and after the procedure. The relation of fluid management through FloTrac/Vigileo monitoring to the extent of the inflammatory response was evaluated. Moreover, we analysed the relation of the inflammatory response to the occurrence of complications. The study was approved by the local Medical and Ethics Committee (Research & Development Department St Antonius Hospital).
A power calculation was performed. Assuming a power of 80% with a given a conventional level of alpha of 0.05, we calculated that, for an expected reduction in fluid administration of 30%, a sample size of 40 patients were needed. Patients were randomised to FloTrac/Vigileo monitoring or standard monitoring with block randomisation and block size of 2.
Patient population
All patients 18 years and older undergoing a HIPEC procedure (independent of the underlying malignancy) from January 2011 to January 2014 were asked to participate. The patients were screened for eligibility to participate in the study by preoperative assessment. Inclusion criteria were age >18 years and undergoing HIPEC procedure irrespective of underlying malignancy. Exclusion criteria were left ventricle ejection fraction under 40%, severe coronary artery disease and unwilling or unable to receive epidural anesthesia. Eligible patients that approved to participate gave written informed consent.
HIPEC procedure
All participants underwent a median laparotomy. Macroscopic debulking was performed first. At the surgeon’s discretion the intestines were anastomosed or partially repaired. If necessary, lesions of diaphragm and peritoneum were resected. The HIPEC-procedure includes 90 minutes of peritoneal lavage with a heated (40–41 °C) chemotherapeutic drug (mitomycin). Extensive operative debulking with peritonectomy and, when needed, multi-organ resections, e.g., liver and spleen, were performed, as described by Sugarbaker et al. (22) and all the latter recommendations (23). The purpose of the cytoreduction was to obtain a macroscopically complete cytoreductive surgery (R1) resection, which means that no macroscopically visible residual tumour was left at the end of the surgical resection. After the cytoreduction, the open perfusion protocol of the abdominal cavity with mitomycin C was performed (24). The inflow temperature of the perfusate was 41–42 °C. As soon as this temperature was reached, mitomycin C was added, 35 mg/m2 body surface area, in three fractions (one half, one fourth, and one fourth of the total dose) with a 30-min dosing interval.
Anesthesia
Before induction of general anesthesia, a median epidural catheter was placed in all patients. Epidural analgesia was initiated before incision with 10 mL of chirocaine 0.25% after which continuous administration was started with bupivacaine 0.125% with sufentanil 1 µg per mL (6 to 10 mL/h). Perioperative antimicrobial prophylaxis was routinely administered in all patients, i.e., 2 grams of cefazoline IV, at time of induction and repeated after 4 hours of surgery. Induction of general anesthesia was performed with propofol (2 mg/kg), fentanyl (3 µg/kg), and atracurium (0.5 mg/kg). After induction, anesthesia was maintained with continuous administration of propofol. A tracheal tube was placed and the patients were ventilated with tidal volumes of 6 to 8 mL per kg body weight. A radial artery catheter and a central venous catheter (right internal jugular vein) were placed after induction of general anesthesia and intubation. All patients received PONV-prophylaxis according to local protocol, i.e., combination of dexamethasone, ondansetron, and haloperidol, when necessary.
After the procedure the patients were admitted to the post-anesthesia care unit (PACU) or intensive care unit (ICU) for at least 24 hours.
The following complications were recorded: mortality, anastomotic dehiscence, renal failure, pneumonia, delirium, pulmonary embolus.
Collection of blood samples
Blood specimens for hemoglobin, leukocytes, and platelet counts were collected into 4.5-mL glass Vacutainer® tubes containing EDTA (Becton Dickinson, Franklin Lakes, USA). Blood samples were collected in 4.5-mL siliconized glass Vacutainer® tubes containing 3.8% trisodium citrate solution (0.105 M) (Becton Dickinson). Samples were collected via a radial artery catheter, centrifuged for 20 minutes at 1,500 ×g, and stored in aliquots at −80 °C until analysis. Blood samples were collected: (I) after placement of radial artery catheter; (II) just before induction of hyperthermia; (III) thirty minutes after hyperthermia; (IV) at the end of hyperthermia; (V) at arrival in the ICU; (VI) after 12; and (VII) after 24 hours postoperatively.
Biochemical analysis
Plasma samples were thawed and analysed for interleukin-1 receptor antagonist (IL-1RA), IL-6, IL-8/CXCL8, IL-10, IL-18, tumor necrosis factor alpha (TNFα), monocyte chemotactic protein 1 (MCP-1), elastase, CRP, and the complement activation product C5a, using multiplex bead assays performed in the Laboratory of Translational Immunology (LTI) of the University Medical Center Utrecht, the Netherlands as described (25,26). For statistical analysis, concentrations below the detection limit were converted to half of the lower limit of detection. Detection limit for all plasma interleukins was less than 1 ng/L. Normal values of IL-8 and IL-6 are <10 ng/L. Normal levels of C5a are <10 ng/mL (27). The detection limit of the CRP assay was 10 ng/L. CRP levels in healthy persons are below 3 mg/L. Mean level of elastase in healthy volunteers is 22 µg/L (28).
When 10% of measurements were below detection limit, the mediator was excluded for further analysis, unless N90% was detected in one specific subgroup.
Statistical analysis
For data storage and statistical analysis, standard computer software (SPSS 24, IBM Corp, Armonk NY) was used. A two-tailed P value <0.05 was considered statistically significant in all tests. Continuous data are presented as mean and standard deviation (SD), if Gaussian distributed and as median and interquartile range (IQR), if not normally distributed. To compare independent continuous variables between groups, a Student t test in case the values followed a Gaussian distribution or the Mann-Whitney U test was conducted otherwise, where appropriate. Categorical variables are given as frequencies and percentages. To compare dichotomous variables between groups, a χ2-test or Fisher’s exact test was used.
Results
Patient characteristics
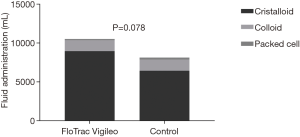
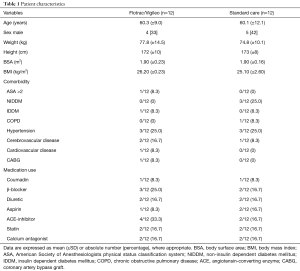
Over a 2-year period, 37 patients undergoing HIPEC surgery gave informed consent to participate in the study. Exclusion criteria were patients under the age of 18 years, left ventricular function below 40%, preoperative arrhythmia, severe coronary artery disease, preoperative use of diuretics and no epidural anaesthesia. Thirteen patients were excluded during the operation in case of terminating surgery for inoperable tumours before starting with HIPEC. Patient characteristics of the 24 patients are shown in Table 1. There were no significant differences between the baseline variables. The total amount of fluid administrated during the first 24 hours was not significantly different between the two groups (10,437±987 vs. 8,135±760 mL, P=0.078, Figure 1), as was the total amount of packed cells (150±170 vs. 250±110 mL, P=0.45).
Full table
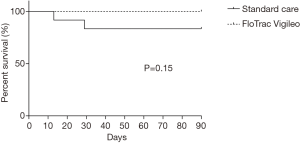
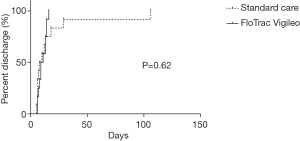
We found no significant difference in 30- and 90-day survival (Figure 2), nor in duration of hospital stay (Figure 3). Complications are shown in Table 2 and were not significantly different between groups. Leucocytes gradually peaked from baseline (6.3×109±2.3×109 cells/L) to 13.0×109±3.1×109 cells/L at t7 (P<0.05).

Full table
Inflammatory markers
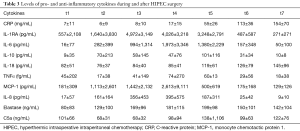
Inflammatory markers were measured at several time points during and after surgery. The samples of one patient were lost. Mean levels of the remaining 23 patients are shown in Table 3 and Figure 4. IL-1RA is an important mediator of the inflammatory response and produced by activated macrophages. We found a significant increase compared with baseline levels at t3, t4 and t5 (baseline 557±2,108 to max 4,972±3,149 pg/mL at t3, normalized at t6). Levels of MCP-1 (monocyte chemoattractant protein 1) showed wide variation with a significant rise at t3 (1,442±2,132 pg/mL) and t5 (500±619 pg/mL) compared to baseline (181±309 pg/mL). Because of a large variation the increase in t4 was not significant (2,613±9,111 pg/mL). C5a, a cleavage product of complement component C5 and a highly inflammatory cytokine, did not show a significant increase during the first 24 hours. In our patients we found a relatively higher baseline mean of C5a (101±66 ng/mL) with a small but significant decrease a t2 (68±31 ng/mL) and t3 (68±32 ng/mL). TNFα levels were not significantly different in the first 24 hours compared with baseline (t1 45±202 fg/mL to max 74±270 fg/mL at t4, ns). IL-6 showed an inflammatory response with a peak at t4, end of hyperthermic phase (16±77 to 1,973±3,346 pg/mL). IL-8 showed the same response as IL-6 (t1 17±57 to t4 395±575 pg/mL). Both cytokines had returned to baseline levels after 12 hours at the ICU. The same response was shown for IL-1RA. IL-10, an anti-inflammatory cytokine showed a slightly slower reaction than IL-1RA with peak levels at t6 (9±35 to 101±116 pg/mL) and returned to baseline levels after 24 hours at the ICU. CRP showed a significant increase from the end of hyperthermia to 24 hours after surgery (t1 7±11 to t7 154±70 pg/mL). Elastase, a neutrophil degradation protein, showed a significant increase at all moments after initiation of surgery (t1 80±83 to max t5 199±98 ng/mL).
Full table
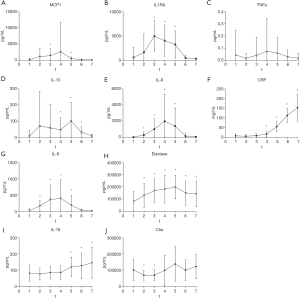
There were no differences in the cytokine levels between the control and intervention group except for C5a. For C5a, levels were not significantly different except at t3 and t4 where levels of the control group were higher (t3; 83±37 vs. 55±22 ng/mL, P=0.46, t4; 147±117 vs. 53±26 ng/mL, P=0.26).
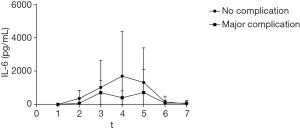
Figure 5 shows the difference in IL-6 levels in patients with and without complications. As shown, we found no difference in IL-6 levels in patients with complications as compared to patients without complications.
Discussion
The aim of the present study was to evaluate if the use of non-invasive CO monitoring with the FloTrac/Vigileo system leads to a more individually tailored fluid administration in patients undergoing HIPEC surgery. Especially in favour of an assumed possible reduction of fluid administration during abdominal surgery with HIPEC by the use of FloTrac/Vigileo power calculation showed that 40 patients would be needed for this study. We did not manage to include such number of patients mainly because many potentially eligible patients did not proceed to HIPEC due to failure of macroscopic tumour reduction. In line with the OPTIMISE trial published after initiation of this study (29), we observed a trend towards higher fluid intake in the intervention group after inclusion of 24 patients. Moreover, patients in the standard care group received more intravenous fluid during than after surgery, while patients in the intervention group received similar volumes during surgery and during the 6 hours postoperatively. The intervention group received more colloid and less crystalloid than the standard care group. Overall administered volumes of intravenous fluid (colloid and crystalloid combined) during the intervention period were similar between groups (intervention, 4,190 mL, vs. usual care, 4,024 mL). Therefore, the null hypothesis could be rejected and by acceptance of the alternative hypothesis with no reduction of fluid administration in the study group the inclusion of patients was stopped.
The complication rate and hospital length of stay were equal in both groups in our study as was found in the OPTIMISE trial. The authors performed a meta-analysis including the data from their study in which they found fewer complications in the intervention group. Furthermore, they found a nonsignificant decrease in hospital, 28- and 30-day mortality. More recently a study was published with high risk patients undergoing cardiac surgery in which they found a reduction in 30-day major complications in patients treated with goal-directed therapy (30).
To our knowledge this is the first study to evaluate the response of IL-1RA, MCP1, C5a, TNFα, IL-6, IL-8, IL-10, IL-18, CRP and elastase at seven moments during and after HIPEC surgery. We found an inflammatory response in all patients undergoing HIPEC surgery. There was a significant increase in the cytokines IL-18, CMP-1, IL-6, IL-8, IL-10 and IL-1RA. Elastase and CRP also showed this increase as expected. We did not find a response in TNFα and C5a. All cytokines showed a large variation between patients. Coccolini et al. (31) also evaluated the response of IL-6 and TNFα in HIPEC surgery patients at two moments (before and after surgery). They also found an increase in IL-6 but no increase in TNFα.
Multiple studies have shown a relation between perioperative IL-6 levels and postoperative complications (21,32,33), while other studies did not find a relation between IL-6 levels and the occurrence of anastomotic leakage after colorectal surgery (34,35). In the present study we found no relation between IL-6 levels and the occurrence of complications. This is in line with the studies of Zielinska-Borkowska and Bilgin (N=157). In contrast, Rettig and colleagues with approximately the same number of patients observed a relation between IL-6 levels on day 1 and the occurrence of postoperative complications in 136 patients undergoing major abdominal surgery (21). Furthermore, the levels of IL-6 were also related to a longer hospital stay. Possibly our study was underpowered to find this relation. A possible explanation may be that the human body reacts in more or less the same way and the inflammatory response does not discriminate between infectious or more organ specific complications, such as myocardial damage.
So, the primary limitation of our study was that the study was prematurely discontinued. However, our hypothesis that this mode of intensive fluid monitoring could positively contribute to a perioperative reduction in the administered volume of infusion fluid during the HIPEC procedure could be rejected early after 24 patients.
In conclusion, the use of non-invasive CO monitoring by means of FloTrac/Vigileo does not lead to more optimal fluid administration in patients undergoing HIPEC surgery. An inflammatory response was observed in all HIPEC surgery patients and there was no difference in this response in patients monitored with the FloTrac/Vigileo system compared with the control group. In this small study we found no relation between the change in inflammatory mediators, such as IL-6 response and the occurrence of complications.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Medical and Ethics Committee (Research & Development department St Antonius Hospital) (No. V.11.332/ R-11.33A).
References
- Simkens GA, van Oudheusden TR, Luyer MD, et al. Serious Postoperative Complications Affect Early Recurrence After Cytoreductive Surgery and HIPEC for Colorectal Peritoneal Carcinomatosis. Ann Surg Oncol 2015;22:2656-62. [Crossref] [PubMed]
- Malfroy S, Wallet F, Maucort-Boulch D, et al. Complications after cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis: Risk factors for ICU admission and morbidity prognostic score. Surg Oncol 2016;25:6-15. [Crossref] [PubMed]
- Newton AD, Bartlett EK, Karakousis GC. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol 2016;7:99-111. [PubMed]
- Rankovic VI, Masirevic VP, Pavlov MJ, et al. Hemodynamic and cardiovascular problems during modified hyperthermic intraperitoneal perioperative chemotherapy. Hepatogastroenterology 2007;54:364-66. [PubMed]
- Esquivel J, Angulo F, Bland RK, et al. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open "coliseum technique". Ann Surg Oncol 2000;7:296-300. [Crossref] [PubMed]
- Raue W, Tsilimparis N, Bloch A, et al. Volume therapy and cardiocircular function during hyperthermic intraperitoneal chemotherapy. Eur Surg Res 2009;43:365-72. [Crossref] [PubMed]
- Mallappallil M, Sabu J, Friedman EA, et al. What Do We Know about Opioids and the Kidney?. Int J Mol Sci 2017;18. [Crossref] [PubMed]
- Kajdi ME, Beck-Schimmer B, Held U, et al. Anaesthesia in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: retrospective analysis of a single centre three-year experience. World J Surg Oncol 2014;12:136. [Crossref] [PubMed]
- Thanigaimani K, Mohamed F, Cecil T, et al. The use of cardiac output monitoring to guide the administration of intravenous fluid during hyperthermic intraperitoneal chemotherapy. Colorectal Dis 2013;15:1537-42. [Crossref] [PubMed]
- Mayer J, Boldt J, Poland R, et al. Continuous arterial pressure waveform-based cardiac output using the FloTrac/Vigileo: a review and meta-analysis. J Cardiothorac Vasc Anesth 2009;23:401-6. [Crossref] [PubMed]
- Benes J, Chytra I, Altmann P, et al. Intraoperative fluid optimization using stroke volume variation in high risk surgical patients: results of prospective randomized study. Crit Care 2010;14:R118. [Crossref] [PubMed]
- Gan TJ, Soppitt A, Maroof M, et al. Goal-directed intraoperative fluid administration reduces length of hospital stay after major surgery. Anesthesiology 2002;97:820-6. [Crossref] [PubMed]
- Venn R, Steele A, Richardson P, et al. Randomized controlled trial to investigate influence of the fluid challenge on duration of hospital stay and perioperative morbidity in patients with hip fractures. Br J Anaesth 2002;88:65-71. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 2018;378:2263-74. [Crossref] [PubMed]
- Auxiliadora-Martins M, Apinages Dos Santos E, Adans Wenzinger D, et al. Perforation of the right ventricle induced by pulmonary artery catheter at induction of anesthesia for the surgery for liver transplantation: a case report and reviewed of literature. Case Rep Med 2009;2009:650982. [Crossref] [PubMed]
- Sakr Y, Vincent JL, Reinhart K, et al. Use of the pulmonary artery catheter is not associated with worse outcome in the ICU. Chest 2005;128:2722-31. [Crossref] [PubMed]
- Boyd KD, Thomas SJ, Gold J, et al. A prospective study of complications of pulmonary artery catheterizations in 500 consecutive patients. Chest 1983;84:245-9. [Crossref] [PubMed]
- Krige A, Bland M, Fanshawe T. Fluid responsiveness prediction using Vigileo FloTrac measured cardiac output changes during passive leg raise test. J Intensive Care 2016;4:63. [Crossref] [PubMed]
- Sangkum L, Liu GL, Yu L, et al. Minimally invasive or noninvasive cardiac output measurement: an update. J Anesth 2016;30:461-80. [Crossref] [PubMed]
- Rettig TC, Verwijmeren L, Dijkstra IM, et al. Postoperative Interleukin-6 Level and Early Detection of Complications After Elective Major Abdominal Surgery. Ann Surg 2016;263:1207-12. [Crossref] [PubMed]
- Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990;50:5790-4. [PubMed]
- Sugarbaker PH, Van der Speeten K. Surgical technology and pharmacology of hyperthermic perioperative chemotherapy. J Gastrointest Oncol 2016;7:29-44. [PubMed]
- Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001;27:365-74. [Crossref] [PubMed]
- Scholman RC, Giovannone B, Hiddingh S, et al. Effect of anticoagulants on 162 circulating immune related proteins in healthy subjects. Cytokine 2018;106:114-24. [Crossref] [PubMed]
- de Jager W, te Velthuis H, Prakken BJ, et al. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin Diagn Lab Immunol 2003;10:133-9. [PubMed]
- Yuan J, Gou SJ, Huang J, et al. C5a and its receptors in human anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. Arthritis Res Ther 2012;14:R140. [Crossref] [PubMed]
- Nuijens JH, Abbink JJ, Wachtfogel YT, et al. Plasma elastase alpha 1-antitrypsin and lactoferrin in sepsis: evidence for neutrophils as mediators in fatal sepsis. J Lab Clin Med 1992;119:159-68. [PubMed]
- Pearse RM, Harrison DA, MacDonald N, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181-90. [Crossref] [PubMed]
- Osawa EA, Rhodes A, Landoni G, et al. Effect of Perioperative Goal-Directed Hemodynamic Resuscitation Therapy on Outcomes Following Cardiac Surgery: A Randomized Clinical Trial and Systematic Review. Crit Care Med 2016;44:724-33. [PubMed]
- Coccolini F, Corbella D, Finazzi P, et al. Time course of cytokines, hemodynamic and metabolic parameters during hyperthermic intraperitoneal chemotherapy. Minerva Anestesiol 2016;82:310-9. [PubMed]
- Szczesny TJ, Slotwinski R, Stankiewicz A, et al. Interleukin 6 and interleukin 1 receptor antagonist as early markers of complications after lung cancer surgery. Eur J Cardiothorac Surg 2007;31:719-24. [Crossref] [PubMed]
- Sanders J, Hawe E, Brull DJ, et al. Higher IL-6 levels but not IL6 -174G>C or -572G>C genotype are associated with post-operative complication following coronary artery bypass graft (CABG) surgery. Atherosclerosis 2009;204:196-201. [Crossref] [PubMed]
- Bilgin IA, Hatipoglu E, Aghayeva A, et al. Predicting Value of Serum Procalcitonin, C-Reactive Protein, Drain Fluid Culture, Drain Fluid Interleukin-6, and Tumor Necrosis Factor-alpha Levels in Anastomotic Leakage after Rectal Resection. Surg Infect (Larchmt) 2017;18:350-6. [Crossref] [PubMed]
- Zielinska-Borkowska U, Dib N, Tarnowski W, et al. Monitoring of procalcitonin but not interleukin-6 is useful for the early prediction of anastomotic leakage after colorectal surgery. Clin Chem Lab Med 2017;55:1053-9. [Crossref] [PubMed]