
The effect of intraoperative fluid administration on outcomes of patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy
Introduction
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is an established treatment for patients with metastatic cancer that has spread to the peritoneum. The procedure involves removing all macroscopically visible tumour, which often necessitates the need for resection of multiple organs. This is then followed by administration of chemotherapy into the abdominal cavity at 42 °C for up to 90 minutes (1). Due to the extensive nature of this procedure, morbidity can be as high as 50% (2).
Strategies and outcomes of intraoperative fluid (IOF) administration have been widely studied in various surgical specialties. Determining the effect of liberal, restrictive or goal directed therapy on patient outcomes has been the focus. Inadequate fluid resuscitation during surgery can expose patients to tissue hypoperfusion and subsequent organ damage. On the other hand, excessive fluid administration can have adverse effects on cardiac and lung function, lead to tissue oedema resulting in organ dysfunction, as well as impair wound healing and coagulation (3). The most recent randomized control trials in major abdominal surgery have demonstrated decreased perioperative morbidity in patients that receive restrictive fluid regimens (4-7).
IOF management in CRS/HIPEC has been less studied compared to other major abdominal surgery. Limited data exists on the effect that various types of fluids have on postoperative patient outcome. Appropriate practices regarding fluid administration are yet to be defined. Thus, our unit aimed to investigate the association between different IOF administration practices and whether or not these influenced postoperative outcomes in patients undergoing CRS/HIPEC.
Methods
Patient selection
Patients who underwent CRS/HIPEC from February 2010 to June 2017 at St George Hospital (Sydney, Australia) were identified. Data was collected from a database at the Peritonectomy Unit of St George Hospital. Patients were thoroughly staged preoperatively by contrast-enhanced computer tomography (CT) of chest, abdomen and pelvis, fluorodeoxyglucose positron emission tomography (PET), and either CT portography or gadoxetate disodium-enhanced (PrimovistTM) magnetic resonance imaging (MRI). All patients were discussed at a multidisciplinary team meeting prior to surgery, which included radiologists, surgeons, medical oncologists, as well as allied health staff to determine suitability for treatment. All patients received three sachets of sodium picosulfate (PicoPrep®) for bowel preparation on the day prior to surgery.
Fluid administration data was retrospectively collected from anaesthetic charts. Those with missing or incomplete anaesthetic charts were excluded. There was no standardized approach to IOF administration among anaesthetists, however, consultant anaesthetists adhered to established practices of fluid resuscitation. Standard monitoring was implemented by anaesthetists across all cases including the use of central venous catheter (CVC), arterial lines and indwelling catheters (IDC). Coagulation status was regularly checked using both formal laboratory assessment and Thromboelastography (ROTEM/TEG). No continuous cardiac output monitoring was used at the time of the study. No patients received epidurals due to concerns regarding coagulopathy.
Because this was regarded as service development by the local research and development committee, formal ethical approval for this retrospective analysis was not required.
Data collection
Preoperative data collected included age, sex, weight, albumin and American Society of Anaesthesiologists (ASA) physical status score. Intraoperative variables collected included volume of crystalloid, 4% albumin, packed red blood cells (PRBCs), fresh frozen plasma (FFP) and cryoprecipitate administered. Operative time, tumour type and tumour volume as depicted by the peritoneal cancer index (PCI) were also recorded. PCI is a score from 0 to 39 to determine the extent of disease within 13 regions of the peritoneal cavity. A score of 0 to 3 is allocated to each region based on the size and confluence of the tumour (8). IOF administration was converted to millilitres per kilogram per hour (mL/kg/h) to account for variations in patient weight and operative time. For certain analyses, fluid administration rates were corrected for PCI to account for variation in fluid administration with increasing disease volume. Patients were divided into high and low transfusion groups for each fluid type according to the median.
Postoperative data collected included length of intensive care unit (ICU) admission, total hospital length of stay (LOS) and patient morbidity according to the Clavien-Dindo classification system (9).
Statistical analysis
Continuous variables were compared using Student’s t-test, Wilcoxon rank-sum, one-way analysis of variance ANOVA, and/or Kruskal-Wallis-tests as appropriate. Where necessary, log- transformation of data was performed to achieve normal distribution. Differences between proportions derived from categorical data were compared using Pearson’s χ2- or Fisher’s exact test where appropriate. Data are reported as median with inter-quartile range (IQR) unless denoted otherwise. To determine the impact of IOF administration patterns on patient outcomes, patients were stratified into low- vs. high-groups according to the fluid-type administered (crystalloid, colloid, blood products, etc.) and split by the cohort median. For LOS data (both ICU and total hospital LOS) uni- and multivariable linear regression modeling was conducted, whereas for factors predicting high-grade (grade 3/4) complications uni- and multivariable logistic regression analysis was performed. All P values <0.05 were regarded as statistically significant and all analyses were performed using R Statistical Packages (10).
Results
Patient demographics
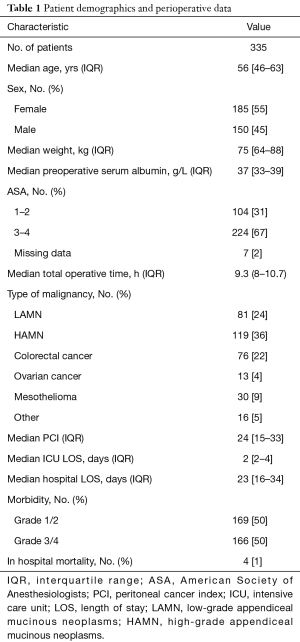
A total of 335 patients who underwent CRS/HIPEC were identified. An overview of patient demographics is provided in Table 1. Of these patients, 185 (55%) were female and 150 (45%) were male. The median age was 56 (IQR, 46–63). The median weight was 75 (IQR, 64–88) kg and most patients [224 (67%)] had an ASA score of 3 or more. Median total operative time was 9.3 (IQR, 8–10.7) hours. The median PCI was 24 (IQR, 15–33). The majority of patients [200 (60%)] had a diagnosis of appendiceal cancer.
Full table
The median hospital LOS and ICU admission was 23 (IQR, 16–34) days and 2 (IQR, 2–4) days respectively. A total of 166 (50%) patients had grade 3/4 complications postoperatively. There were 4 in-hospital deaths (1% procedure specific mortality rate).
Fluids administered
Median total volume (TV) of fluid administered per case was 11,050 (IQR, 8,500–15,200) mL which when corrected for weight and operative time, equated to a median rate of 17 (IQR, 12–23) mL/kg/h. Median crystalloid and albumin (CA) transfused was 7,500 (IQR, 6,000–9,000) mL at a rate of 11 (IQR, 9–14) mL/kg/h. The median TV of blood products (BPs) transfused (including PRBCs, FFP and cryoprecipitate) was 3,300 (IQR, 1,700–6,400) mL at a rate of 5 mL/kg/h. Patients that received increasing volumes of BPs were likely to receive less CA and vice versa (data not shown).
The effect of TV transfused on patient outcomes
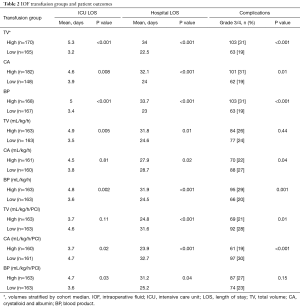
Totally, 170 (51%) patients were considered in the high TV group and 165 (49%) in the low TV group. Those who received larger volumes of IOFs had longer hospital LOS (mean, 34 vs. 22.5 days; P<0.001), extended ICU admission (mean, 5.3 vs. 3.2 days; P<0.001) and a 12% increase in grade 3/4 complications (P<0.001) (Table 2). Even when corrected for patient weight and operative time, higher TVs administered still resulted in longer hospital LOS (mean, 31.8 vs. 24.6 days; P=0.01) and longer ICU admission (mean, 4.9 vs. 3.5 days; P=0.005), but there was no statistically significant difference in rates of grade 3/4 complications (mean, 84 vs. 77; P=0.44).
Full table
The effect of crystalloid & albumin transfusions on patient outcomes
Totally, 182 (55%) patients were deemed high volume and 148 (45%) low volume. Patients who received a high volume of CA demonstrated an increased hospital LOS (mean, 32.1 vs. 24 days; P<0.001), longer ICU admission (mean, 4.6 vs. 3.9 days; P=0.008) and a 12% increase in grade 3/4 complications (P=0.01). However, when corrected for weight, operative time and PCI, the lower volume cohort had increased hospital LOS (mean, 32.7 vs. 23.9 days; P<0.001), prolonged ICU admission (mean, 4.7 vs. 3.7 days; P=0.02) and an 11% increase in grade 3/4 complications (P<0.001).
The effect of BP transfusion on patient outcomes
A total of 163 (50%) patients were in the high-volume cohort and 163 (50%) in the low volume cohort. Higher transfusion volumes of BPs resulted in longer hospital LOS (mean, 33.7 vs. 23 days; P<0.001), extended ICU admission (mean, 5 vs. 3.4 days; P<0.001) and an 11% increase in grade 3/4 complications (P<0.001). When corrected for weight and operative time, higher BP transfusion rates resulted in longer hospital LOS (mean 31.9 vs. 24.5 days; P<0.001), prolonged ICU admission (mean, 4.8 vs. 3.6 days; P=0.002) and a 9% increase in grade 3/4 complications (P=0.001). Even when corrected for patient PCI, the administration of higher volumes of BPs resulted in longer hospital LOS (mean, 31.2 vs. 25.2 days; P=0.04) and longer ICU admission (mean, 4.7 vs. 3.6 days; P=0.03).
Analysis of factors contributing to total hospital and ICU LOS and postoperative morbidity
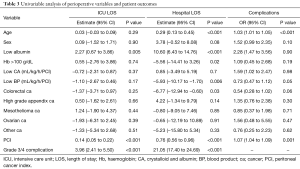
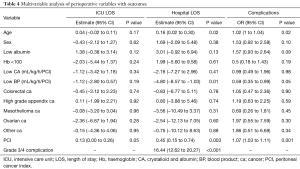
Regression analysis was performed for three outcomes; total hospital LOS, ICU LOS and development of postoperative complications. The results of the univariable analysis are provided in Table 3. On multivariable analysis (Table 4) age, PCI, BP transfusion and the occurrence of grade 3/4 complications were associated with increased hospital LOS. Only PCI was associated with extended ICU admission on multivariable analysis. With regards to complications, age, increased BP transfusions and PCI were independently associated with increased grade 3/4 complications on multivariable analysis.
Full table
Full table
Discussion
The optimal approach to fluid administration during surgery has been extensively studied. However, limited data exists for CRS/HIPEC and a recommended approach to fluid administration in this type of surgery is lacking. Due to the unique physiological changes that occur with CRS/HIPEC, it is uncertain whether evidence-based strategies of fluid administration in general abdominal surgery also apply to CRS/HIPEC. Large abdominal access, lengthy operative time and extensive debulking predispose patients to hypothermia and enormous protein loss. At the time of HIPEC, the rise in body temperature results in increased metabolic rate and oxygen demands (11-14). These homeostatic changes affect fluid balance and coagulopathy and thus provide anaesthetists with a unique challenge in maintaining balanced fluid resuscitation.
Traditionally, a more aggressive approach to fluid resuscitation during surgery was applied. This was in part based on the premise that a preoperative patient was hypovolaemic as a result of fasting with continuing ongoing losses, as well as concepts of replacing insensible fluid losses during surgery such as ‘third spacing’ (15). However, in light of emerging data there has been a shift away from liberal and toward restrictive regimens. The adverse effects of fluid overload on morbidity date back to 1990 (16). Excessive fluid resuscitation has been associated with pulmonary oedema, prolonged mechanical ventilation and ileus, cardiac dysfunction, impaired wound healing and coagulation (3,17). Our study also demonstrated similar unfavourable effect with greater IOF administration increasing patients’ ICU admission, hospital LOS and grade 3/4 complications.
The paradigm shift supporting a restrictive approach to fluid resuscitation during surgery came in the wake of a number of studies demonstrating favourable patient outcomes with less fluid administration (4-6,18). However, none of these studies have specifically looked at CRS/HIPEC.
Currently, a limited number of studies exist examining IOF administration on patient outcomes in CRS/HIPEC. Recently, Eng et al. (19) analysed 133 patients undergoing CRS/HIPEC. Using the comprehensive complication index (CCI) (20) as a marker for patient outcomes they demonstrated a significant increase in perioperative morbidity with greater IOF administration. Patients that received an IOF rate of greater than or equal to 15.7 mL/kg/h developed a 43% increase in CCI compared to those whose rate was lower than 15.7 mL/kg/h. Another study looking at CRS/HIPEC in ovarian cancer showed increased patient morbidity with perioperative fluid excess. In particular, greater IOF administration was independently associated with surgical site infection (21). These studies are consistent with our findings that increased IOF administration over 17 mL/kg/h results in adverse patient outcomes in CRS/HIPEC.
However, our findings offer the novel perspective of assessing the types of fluid administered separately and its effect on patient outcomes. Specifically, our study demonstrated the deleterious effect increased BP transfusion has on patient outcomes. Even when corrected for PCI, increased administration of BPs resulted in longer hospital LOS and ICU admission. On multivariable analysis, BP transfusion was independently associated with increased grade 3/4 complications. In a previous study from our unit, a review of 936 patients undergoing CRS/HIPEC by Saxena et al. (22) also demonstrated the negative impact of intraoperative blood transfusion on patient outcome. These authors only examined the effect of PRBC and found that with massive allogenic blood transfusion (defined as greater than or equal to 5 units), patient’s experienced prolonged hospital LOS, extended ICU admission and increased grade 3/4 complications. Single centre studies by Kajdi et al. (23) and Cascales-Campos et al. (24) have also shown blood transfusions to be an independent risk factor for grade 3/4 complications in patients undergoing CRS/HIPEC. The adverse effect of BP transfusion on patient outcomes has been well established in surgical literature (25-31) and our findings provide further evidence of this in the context of CRS/HIPEC.
The adverse effects evident with greater intraoperative BP transfusion could explain the finding of increased hospital LOS, ICU admission and grade 3/4 complications in the low volume CA transfusion group. We noted that with increasing PCI, the proportion of BPs to CA administered was greater. Because these patients were receiving greater amount of BPs, they in turn received less CA. Thus, this variation in proportionality could explain the longer hospital LOS, extended ICU admission and increased grade 3/4 complications in the low CA transfusion group.
In regards to liberal and restrictive fluid regimens during surgery, goal-directed therapy (GDT) has emerged as a viable approach to fluid resuscitation. The use of GDT has demonstrated fewer complications in surgery (32). In major abdominal surgery, GDT is associated with significant reduction in patient morbidity and improved outcomes (33-35). Recently, a prospective, randomized controlled study comparing goal directed and standard fluid therapy was conducted by Colantonio et al. (36) in patients undergoing CRS/HIPEC. Eighty patients were randomized to goal-directed (n=38) and standard (n=42) fluid therapy arms. The control group received a baseline fluid rate of 4 to 10 mL/kg/h whilst the GDT group received a baseline fluid rate of 4 mL/kg/h with boluses of colloid to maintain a set cardiac index threshold. Those in the GDT group received a significantly lower amount of fluid than the control group. The incidence of major abdominal and systemic complications was less in the GDT group and hospital LOS was also reduced. These findings show the benefit of GDT in CRS/HIPEC and thus a potential approach to future fluid resuscitation. It was interesting to note that in this study, the baseline fluid rate of 4 to 10 mL/kg/h was significantly lower than our median fluid rate of 17 mL/kg/h. This discrepancy could be explained by the greater volume of high PCI procedures undertaken at our institution, the inclination of anaesthetists to over resuscitate in order to avoid hypovolaemia and subsequent kidney injury, and the small number of patients in the GDT arm of the randomized control trial.
Our findings are consistent with other studies that demonstrate increased IOF administration results in unfavourable patient outcomes. We have shown that with increasing IOF administration patients are subject to prolonged hospital LOS, extended ICU admission and more grade 3/4 complications. In particular, our study highlighted the deleterious effect BPs has on patient outcomes.
Limitations
Limitations of this study included its retrospective design which did not allow us to establish a causal effect between fluid administration and patient outcome. Pre-operative patient data that was not captured which could influence peri-operative fluid balance included presence of ascites, ostomies and adherence to bowel preparation prior to surgery. Regarding anaesthetic data, even though vasoactive support is minimally used at our institution during CRS/HIPEC, it can affect fluid resuscitation and was not presented in our data. Although not mentioned in our study, varying chemotherapeutic agents and types of cancer may also influence fluid homeostasis and will be subject to future investigation at our institution. The authors recognize that fluid resuscitation in the post-operative period, especially in the first 24 hours, could potentially influence patient outcomes and was not recorded or subject to analysis in the current study. Finally, although global recording of complications through the Clavien-Dindo classification system were conducted, the specific type of complications was not described.
Conclusions
Increasing IOF administration is associated with an increase in patient morbidity in CCRS/HIPEC. Greater use of IOF results in longer hospital LOS, longer ICU admission and increased grade 3/4 complications. This is particularly evident with increasing use of BPs. Designing fluid protocols that favour a more restrictive approach may reduce postoperative patient morbidity in those undergoing CRS/HIPEC. We believe the presented data allows for a more informed discussion about the potential effects of different fluid administration strategies on short-term patient outcomes and may provide the rationale for future prospective studies into this topic.
Acknowledgements
The authors would like to thank the Australian Government, as this project was conducted in part with the Australian Government Research Training Program Scholarship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Because this was regarded as service development by the local research and development committee, formal ethical approval for this retrospective analysis was not required
References
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [Crossref] [PubMed]
- Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754-63. [Crossref] [PubMed]
- Holte K, Sharrock NE, Kehlet H. Pathophysiology and clinical implications of perioperative fluid excess. Br J Anaesth 2002;89:622-32. [Crossref] [PubMed]
- Abraham-Nordling M, Hjern F, Pollack J, et al. Randomized clinical trial of fluid restriction in colorectal surgery. Br J Surg 2012;99:186-91. [Crossref] [PubMed]
- Brandstrup B, Tonnesen H, Beier-Holgersen R, et al. Effects of intravenous fluid restriction on postoperative complications: comparison of two perioperative fluid regimens: a randomized assessor-blinded multicenter trial. Ann Surg 2003;238:641-8. [Crossref] [PubMed]
- Nisanevich V, Felsenstein I, Almogy G, et al. Effect of intraoperative fluid management on outcome after intraabdominal surgery. Anesthesiology 2005;103:25-32. [Crossref] [PubMed]
- Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus Liberal Fluid Therapy for Major Abdominal Surgery. N Engl J Med 2018;378:2263-74. [Crossref] [PubMed]
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 1996;82:359-74. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. Available online: https://www.gbif.org/tool/81287/r-a-language-and-environment-for-statistical-computing
- Esquivel J, Angulo F, Bland RK, et al. Hemodynamic and cardiac function parameters during heated intraoperative intraperitoneal chemotherapy using the open “coliseum technique”. Ann Surg Oncol 2000;7:296-300. [Crossref] [PubMed]
- Kanakoudis F, Petrou A, Michaloudis D, et al. Anaesthesia for intra-peritoneal perfusion of hyperthermic chemotherapy. Haemodynamic changes, oxygen consumption and delivery. Anaesthesia 1996;51:1033-6. [Crossref] [PubMed]
- Schmidt C, Moritz S, Rath S, et al. Perioperative management of patients with cytoreductive surgery for peritoneal carcinomatosis. J Surg Oncol 2009;100:297-301. [Crossref] [PubMed]
- Vorgias G, Iavazzo C, Mavromatis J, et al. Determination of the necessary total protein substitution requirements in patients with advanced stage ovarian cancer and ascites, undergoing debulking surgery. Correlation with plasma proteins. Ann Surg Oncol 2007;14:1919-23. [Crossref] [PubMed]
- Doherty M, Buggy DJ. Intraoperative Fluids: How Much Is Too Much? Surv Anesthesiol 2013;57:53-4. [Crossref]
- Lowell JA, Schifferdecker C, Driscoll DF, et al. Postoperative fluid overload: not a benign problem. Crit Care Med 1990;18:728-33. [Crossref] [PubMed]
- Lobo DN, Macafee DA, Allison SP. How perioperative fluid balance influences postoperative outcomes. Best Pract Res Clin Anaesthesiol 2006;20:439-55. [Crossref] [PubMed]
- Piljic D, Petricevic M, Piljic D, et al. Restrictive versus Standard Fluid Regimen in Elective Minilaparotomy Abdominal Aortic Repair-Prospective Randomized Controlled Trial. Thorac Cardiovasc Surg 2016;64:296-303. [PubMed]
- Eng OS, Dumitra S, O’Leary M, et al. Association of Fluid Administration With Morbidity in Cytoreductive Surgery With Hyperthermic Intraperitoneal Chemotherapy. JAMA Surg 2017;152:1156-60. [Crossref] [PubMed]
- Slankamenac K, Graf R, Barkun J, et al. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg 2013;258:1-7. [Crossref] [PubMed]
- Desale MG, Tanner EJ, Sinno AK, et al. Perioperative fluid status and surgical outcomes in patients undergoing cytoreductive surgery for advanced epithelial ovarian cancer. Gynecol Oncol 2016. [Epub ahead of print]. [PubMed]
- Saxena A, Valle SJ, Liauw W, et al. Allogenic Blood Transfusion Is an Independent Predictor of Poorer Peri-operative Outcomes and Reduced Long-Term Survival after Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: a Review of 936 Cases. J Gastrointest Surg 2017;21:1318-27. [Crossref] [PubMed]
- Kajdi ME, Beck-Schimmer B, Held U, et al. Anaesthesia in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy: retrospective analysis of a single centre three-year experience. World J Surg Oncol 2014;12:136. [Crossref] [PubMed]
- Cascales-Campos PA, Lopez-Lopez V, Munoz-Casares FC, et al. Morbidity and mortality outcomes after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in patients aged 75 years and over: Spanish group of peritoneal cancer surgery (GECOP) multicenter study. Surg Oncol 2016;25:111-6. [Crossref] [PubMed]
- Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg 2012;256:235-44. [Crossref] [PubMed]
- Bernard AC, Davenport DL, Chang PK, et al. Intraoperative transfusion of 1 U to 2 U packed red blood cells is associated with increased 30-day mortality, surgical-site infection, pneumonia, and sepsis in general surgery patients. J Am Coll Surg 2009;208:931-7, 937.e1-2; discussion 938-9.
- Chalfin HJ, Liu JJ, Gandhi N, et al. Blood Transfusion is Associated with Increased Perioperative Morbidity and Adverse Oncologic Outcomes in Bladder Cancer Patients Receiving Neoadjuvant Chemotherapy and Radical Cystectomy. Ann Surg Oncol 2016;23:2715-22. [Crossref] [PubMed]
- Ferraris VA, Davenport DL, Saha SP, et al. Surgical outcomes and transfusion of minimal amounts of blood in the operating room. Arch Surg 2012;147:49-55. [Crossref] [PubMed]
- Halabi WJ, Jafari MD, Nguyen VQ, et al. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg 2013;206:1024-32; discussion 1032-3. [Crossref] [PubMed]
- Houbiers JG, van de Velde CJ, van de Watering LM, et al. Transfusion of red cells is associated with increased incidence of bacterial infection after colorectal surgery: a prospective study. Transfusion 1997;37:126-34. [Crossref] [PubMed]
- Postlewait LM, Squires MH, Kooby DA, et al. The relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer: a multi-institutional study of 456 patients. HPB (Oxford) 2016;18:192-9. [Crossref] [PubMed]
- Giglio MT, Marucci M, Testini M, et al. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth 2009;103:637-46. [Crossref] [PubMed]
- Donati A, Loggi S, Preiser JC, et al. Goal-directed intraoperative therapy reduces morbidity and length of hospital stay in high-risk surgical patients. Chest 2007;132:1817-24. [Crossref] [PubMed]
- Noblett SE, Snowden CP, Shenton BK, et al. Randomized clinical trial assessing the effect of Doppler-optimized fluid management on outcome after elective colorectal resection. Br J Surg 2006;93:1069-76. [Crossref] [PubMed]
- Wakeling HG, McFall MR, Jenkins CS, et al. Intraoperative oesophageal Doppler guided fluid management shortens postoperative hospital stay after major bowel surgery. Br J Anaesth 2005;95:634-42. [Crossref] [PubMed]
- Colantonio L, Claroni C, Fabrizi L, et al. A randomized trial of goal directed vs. standard fluid therapy in cytoreductive surgery with hyperthermic intraperitoneal chemotherapy. J Gastrointest Surg 2015;19:722-9. [Crossref] [PubMed]


