
Dose escalated concurrent chemo-radiation in borderline resectable and locally advanced pancreatic cancers with tomotherapy based intensity modulated radiotherapy: a phase II study
Introduction
Pancreatic cancer has the highest mortality rates among all the gastrointestinal cancers with a 5-year survival of less than 5% (1,2). Resectable pancreatic cancers account for only 20–25% of all cases, with the rest being borderline resectable, locally advanced or metastatic (3). Although surgery is the standard of care for pancreatic cancer, surgery alone even in resectable cases offers limited survival of about 20–24 months (4,5). Since majority of patients fail distally (6), adjuvant chemotherapy has been added to surgery with survival benefit (7). Compliance to adjuvant therapy is poor, with about 50% not receiving any adjuvant treatment due to post op complications or poor performance status (8,9). The feasibility of upfront surgery with negative margins in borderline and locally advanced cases is less. Hence, neoadjuvant approaches involving radiation and chemotherapy have been tried with the intent of optimal downstaging, sterilizations of margins, control of micro metastatic disease, improving survival and avoidance of surgery in patients developing metastasis during the neoadjuvant therapy.
There is no consensus on the optimal neoadjuvant approach and sequencing of modalities for borderline resectable pancreatic cancer (BRPC). Multiagent chemotherapy (FOLFIRINOX), chemotherapy doublets, chemoradiation and SBRT have been tried (10-13). In BRPC, downstaging achieved by chemoradiation results in decreased requirement of complex vascular surgery and improved R0 resection rates. In locally advanced pancreatic cancer (LAPC), chemotherapy is the standard of care. The LAP-07 trial has led to decline in use of chemoradiation in LAPC (14). It showed non-inferiority of chemotherapy alone compared to chemotherapy followed by chemoradiation. Chemoradiation was associated with better local control and progression free survival (PFS), without overall survival (OS) benefit.
Dose escalation from conventional doses of 50 to 67–75 Gy using intensity modulated radiotherapy (IMRT) has shown to improve outcomes in some series (12,15,16). This prospective study was undertaken to assess the response and outcomes of patients with BRPC and LAPC treated with dose escalated neoadjuvant IMRT.
Methods
This is a prospective phase II study, approved by the Institutional review board and human ethics committee. All newly diagnosed non-metastatic BRPC and LAPC patients, staged using contrast enhanced triphasic computed tomography scans (CECT) abdomen and thorax, were eligible for the study. National comprehensive cancer network (NCCN) criteria version 2.2.12 was used to classify patients to BRPC and LAPC. All patients with biopsy proven adenocarcinoma, more than 18 years of age with Eastern Cooperative Oncology Group (ECOG) performance status of 0–2 with normal hematological, renal and hepatic functions (serum bilirubin <3 mg/dL) were considered for the study. Patients having retroperitoneal nodes or distant metastasis on CECT and who had received any prior radiation or chemotherapy were excluded.
All the patients underwent planning positron emission tomography (PET) with CECT in supine position on a flat couch. If PET scan did not detect any further metastatic disease, then the images were used for planning with IMRT. Gross tumor volume (GTV) was delineated on CECT/PET scan which included the primary and involved nodes. The clinical target volume (CTV) was generated by giving margin for the microscopic disease to the GTV. The dose prescribed to GTV was 57 Gy over 25 fractions in 5 weeks whereas the CTV was planned to receive 45 Gy over 25 fractions. The dose constraint to organs at risk (OAR) was as follows: duodenum: V 50 Gy less than <10% and V 40 Gy less than 35%, small bowel: mean dose of <45 Gy; kidney: mean dose <18 Gy to both kidneys and liver: V35 Gy less than 35%. All the patients were treated with IMRT with daily image guidance using tomotherapy. Both BRPC and LAPC were planned for chemoradiation as the initial treatment modality. Concurrent chemotherapy was given weekly with Inj. Gemcitabine to a dose of 300 mg/m2. Adjuvant chemotherapy was recommended to all patients who were operated and inoperable patients continued on palliative chemotherapy.
Response and toxicity evaluation
The patients who completed treatment were evaluated for response at 6 weeks post completion of IMRT using triphasic CECT along with PET and were considered for the surgery. Response assessment was done using RECIST criteria. Toxicity was assessed weekly using National Cancer Institute Common Toxicity Criteria (CTC v 3) during entire course of treatment. Thereafter, all the patients were followed up every 3 months for 2 years and then every 6 months for 3 years with clinical examination and CA 19-9. Imaging was done as indicated clinically.
Surgical procedure
Patients considered suitable for R0 resection on triphasic CECT were planned for surgery. Those found to have metastatic disease on response evaluation PET-CECT were excluded and treated with palliative chemotherapy. Surgical procedure planned was Whipples procedure with vascular resection with lymph node clearance.
Statistics
The study was planned with a primary end point to assess the R0 resection rate in the BRPC and LAPC patients undergoing dose escalated neoadjuvant chemoradiation. The secondary end points were to assess radiological response, acute and late toxicities, loco-regional control and survival. The OS and PFS was estimated using Kaplan Meier method and comparison was done using the log-rank test. OS and PFS were estimated from the date of diagnosis to date of death from any cause and development of progression (local, regional, distant or death) whichever occurred earlier respectively. The quantitative variables were compared between BRPC and LAPC using student t-test or Mann Whitney U test depending on the normality. The quantitative variables were compared pre and post treatment using paired t-test or Wilcoxon signed rank test depending on the normality. A P value of <0.05 was considered statistically significant.
Results
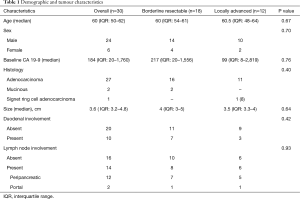
From December 2008 to July 2011, 30 patients were accrued after obtaining written informed consent. Eighteen patients (60%) were BRPC and the rest 12 patients (40%) were LAPC. The median age of entire cohort was 60 [interquartile range (IQR): 50–62] years. Majority were males (24 patients, 80%). The median baseline CA 19-9 was 184 (IQR: 20–1,760). There was no difference in CA 19-9 values between BRPC and LAPC (P=0.76). Table 1 shows the demographic and tumour characteristics. The diagnosis was histologically confirmed in all cases by FNAC/biopsy; majority being adenocarcinoma. Duodenal involvement was seen in 10 patients. Fourteen patients had lymph node involvement; peri-pancreatic and portal nodes. The median pretreatment SUVmax for the primary was 8 (IQR: 5.5–10.5). The nodal status was confirmed on PET with 11 patients having lymph node involvement; 3 patients had no uptake in the enlarged peri-pancreatic nodes.
Full table
Response and toxicity
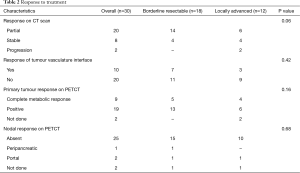
The response assessment CECT scan was done in all patients and showed partial response in 20 (67%) and stable disease in 8 (26%). Two LAPC patients progressed; one had peritoneal metastases and another liver metastases. The median tumour size post treatment was 2.5 (IQR: 1.8–4) cm and there was significant reduction in the primary tumour size (P≤0.001). The change in tumour vasculature interface for the vessels [superior mesenteric artery (SMA), superior mesenteric vein (SMV), portal vein (PV), hepatic artery (HA), and celiac artery (CA)] was seen in 10 patients, however it was statistically significant only for PV (P=0.04). Response assessment PET was done in 28 patients (2 had progressed prior to PET). The median CA 19-9 post treatment was 42 (IQR: 13.5–528.5). The median post treatment SUVmax of the primary was 3 (IQR: 0.25–6.2) and there was significant reduction in the SUVmax compared to pretreatment (P≤0.001). Complete metabolic response (CMR) was seen in 9 patients (30%), decrease in SUVmax in 12 patients and complete nodal response in 25 patients (83%). There was no difference between BRPC and LAPC in terms of response (Table 2). All patients tolerated the treatment well. Grade 1 leucopenia was seen in 1 patient and 3 patients had grade 1 thrombocytopenia.
Full table
Surgical details
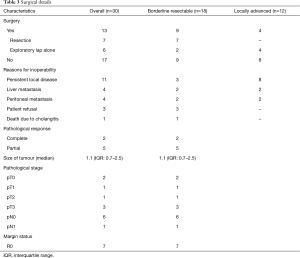
Thirteen patients explored for surgery. Whipple’s procedure with lymph node clearance was done in 7 patients; all were BRPC. None of the LAPC patients were operated. Rest of the 6 patients were deemed unresectable (3 patients-encasing vessels and inoperable) or metastatic (3 patients had peritoneal disease). Details are given in Table 3. Pathological CR was seen in 2 patients. Six patients had negative nodes (pN0, 85.5%). All 7 patients, the margins were negative (R0, 100%). All operated patients received adjuvant chemotherapy (single agent gemcitabine for 6 cycles). Among the operated patients, 2 patients developed postop complications; one patient developed postoperative bile collection and another wound infection (grade 1 Clavien Dindo). None of the inoperable patients developed late gastrointestinal (GI) toxicity in the form of bleeding/malena, ulceration, obstruction or stricture. There was no correlation between PET response and operability (P=0.95).
Full table
Outcomes
The median follow up of entire cohort was 16.1 (IQR: 9–23.4) months and those of surviving patients were 85 (IQR: 64.5–85.8) months. Among BRPC, only 3 patients were alive at last follow up. The median follow up of all BRPC patients was 18.5 (IQR: 9.5–36) months and those of surviving patients were 85 (IQR: 64.5–85.8) months. All the LAPC patients were dead at last follow up and their median follow up was 13.6 (IQR: 9–20) months.
BRPC
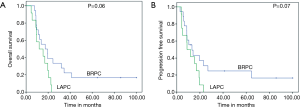
Three patients were alive at last follow up (all of whom underwent R0 resection). The median OS was 17.3 (95% CI: 5.2–29.4) months with 1- and 2-year OS of 61% and 33% respectively (Figure 1A). Thirteen patients died of disease progression; 5 of locoregional progression and 8 of distant metastases (liver, 4 patients; peritoneum, 4 patients). The median PFS was 13.1 (95% CI: 6.2–20) months with 1- and 2-year PFS of 55% and 24% respectively (Figure 1B). The median OS who underwent R0 resection was 35.5 (95% CI: 27.8–41) months with 1-, 2- and 5-year OS of 85%, 57% and 42% respectively. Their median PFS was 27 (95% CI: 1–60) months with 1-, 2- and 5-year PFS of 71%, 42% and 28% respectively. Both OS and PFS were statistically higher compared to inoperable (Figure 2A,B). There was no correlation between PET response and OS (P=0.25) or PFS (P=0.63).
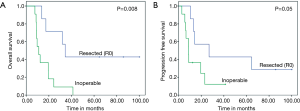
LAPC
The median OS was 11.8 (95% CI: 1.9–21.7) months with 1- and 2-year OS of 50% and 0% respectively (Figure 1A). All patients died due to disease; 8 patients died of persistent/progressive locoregional disease and 4 of distant metastases (liver, 3 patients; peritoneum, 1 patient). The median PFS was 8.8 (95% CI: 3.5–14) months with 1- and 2-year PFS of 41.7% and 0% respectively (Figure 1B). There was no difference in median pre CTRT SUVmax values (7 vs. 9, P=0.32) or post CTRT SUVmax values (2.3 vs. 5, P=0.16) among progression type groups (local/locoregional alone vs. distant).
Discussion
Chemoradiation is a part of multimodality treatment in BRPC and LAPC. In this study, we evaluated the response and outcomes of BRPC and LAPC with dose escalated IMRT and showed that over 80% of patients had partial response or stable disease with dose escalated IMRT. The 39% of BRPC were operated with R0 margin status. The outcomes of those operated among BRPC were superior to the inoperable.
Post treatment imaging CECT may be used to rule out local or distant progression (to avoid surgery) and for planning surgery. CECT or MRI is not reliable for predicting resectability after neoadjuvant treatment (17,18). Dramatic response following chemoradiation radiologically is uncommon even in the event of histological response due to the dense stroma of the pancreatic cancer (18). The relationship with blood vessels rarely changes (19). Katz et al. evaluated the response of BRPC following chemoradiation with CECT and showed that 69% had stable disease, 12% partial response and only 1 patient was down staged to resectable status (20). Sixty-six percent underwent successful surgery with 95% achieving a negative margin. No change in the relationship between tumour and vessels was seen. However, only 1 patient had a positive margin. They also confirmed that RECIST criteria are not appropriate for assessing response in BRPC. In the present study, 67% had partial response and 26% stable disease. There was significant reduction in size of primary and 10 patients showed a change in tumour and vessel interface. Ferrone et al. evaluated neoadjuvant therapy with FOLFIRINOX in BRPC and LAPC and showed that despite about 85% having partial/stable disease on post treatment imaging and lack of clear plane around vessels, 92% of them underwent R0 resection (19). Following neoadjuvant treatment, radiologically there may not be significant response. Surgical exploration must be contemplated in all patients in the absence of progression (local or distant) (17).
PET CECT is commonly used to assess response in solid tumours. FDG PET has been used in pancreatic cancer to assess response following chemoradiation. Chang et al. evaluated the prognostic value of post treatment PETCT in LAPC (21). Patients with baseline SUVmax of <3.5% or >60% decrease in SUVmax post treatment had better OS (41 vs. 16 months). Choi et al. showed that PET responders had complete surgical resection and better survival (22). In our study, CMR was seen in 9 patients and reduction in SUV value in 12 patients. However this was not predictive of better OS or PFS. Surgery was done in only 5 patients who were PET responders and PET response did not correlate with operability. Wilson et al. evaluated PET parameters post chemoradiation (CRT) and correlated with disease progression (local vs. metastatic group) (23). The SUVmax post CRT was significantly lower. A low pre CRT SUVmax value was predictive of local disease at follow up. Higher Post CRT SUVmax value was seen in metastatic group. In the present study, there was significant reduction in the SUVmax compared to pretreatment. There was no difference in median pre CTRT SUVmax values (7 vs. 9, P=0.32) or post CRT SUVmax values (2.3 vs. 5, P=0.16) among progression type groups (local vs. distant).
Neoadjuvant CRT is associated with improved survival outcomes in BRPC. Katz et al. showed improved survival in BRPC who underwent surgery following chemoradiation (33 vs. 12 months) compared to those who did not undergo resection (20). Lee et al. evaluated the outcomes of neoadjuvant CRT followed by surgery vs. surgery alone in BRPC (24). There was improvement in R0 resection rates following CRT (93.3% vs. 71.4%, P=0.03). The disease specific survival was higher in CRT group compared to surgery alone and no surgery (31 vs. 21.3 vs. 19.5 months, P=0.006). There was also significant improvement in disease free survival (P=0.05). Jang et al. conducted randomized trial comparing neoadjuvant chemo radiation with gemcitabine versus upfront surgery in BRPC. Neoadjuvant CRT was associated with improved R0 resection (52% vs. 26%, P=0.004), median survival (21 vs. 12 months, P=0.02) and 2-year OS (40.7% vs. 26%, P=0.02) (25). In the present study, there was significant improvement in OS and PFS among those resected vs. not resected.
In the present study, none of the LAPC patients were resected. However, 8 patients (68%) continued to have local disease alone till death and 4 developed distant metastases. In LAPC, there is a subset of patients who tend to have localized disease with no propensity for systemic spread even after chemotherapy. 30% have only local progression on autopsy (26). DPC 4 gene status has the propensity to identify such patients (26). These subsets of patients are the ones most likely to benefit from radiation. Chemoradiation has been used in LAPC. 2 randomized trials with conventional chemoradiation have failed to show any survival advantage (14,27). However, there was improvement on DFS and LC. The lack of survival benefit was due to systemic progression during the conventional chemoradiation over 4–5 weeks. Dose escalated IMRT in LAPC has shown improved outcomes. In the study by Krishnan et al., 47 LAPC patients treated with induction chemotherapy followed by dose escalated IMRT had improved median OS (17.8 months) with 2- and 3-year OS of 36% and 31% respectively. The median loco-regional recurrence free survival was also improved (10.2 months) (28).
This study has many limitations. This study was conducted in 2008–2011 and both BRPC and LAPC underwent chemoradiation first, which delayed the systemic chemotherapy. This trial design is outdated compared to what is currently considered by most clinical trial groups and does not reflect our current treatment practice. Most trials today are using chemotherapy first, followed by chemo-radiation. Since patients in pancreatic cancer are at risk of distant metastases, systemic chemotherapy is offered first. All patients received gemcitabine based chemotherapy alone. None of the patients received FOLFIRINOX or gemcitabine-nab paclitaxel which have shown to be more effective with higher response rates (29-31). The standard chemotherapy should be at least doublet or FOLFIRINOX and Gemcitabine alone is no longer considered adequate therapy. Complex vascular resections particularly arterial resections were not done in any case. This explains the lower rates of surgery in our study. Further the surgical planning was based on radiological downstaging and the patients with no downstaging were not explored for surgery. This study has few strength. It is a prospective study evaluating dose escalated IMRT in pancreatic cancer. All patients underwent good quality CECT as per pancreas protocol and the response was uniformly reported by a single radiologist. PETCT showed significant reduction in SUVmax post treatment.
Conclusions
Dose escalated IMRT in pancreatic cancer allows tumour downstaging. There was significant R0 resections in 39% of BRPC. Complete pathological response was seen in 2 patients with 85% having complete nodal response.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional review board and human ethics committee.
References
- Herszenyi L, Tulassay Z. Epidemiology of gastrointestinal and liver tumors. Eur Rev Med Pharmacol Sci 2010;14:249-58. [PubMed]
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Li D, Xie K, Wolff R, et al. Pancreatic cancer. Lancet 2004;363:1049-57. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 2013;310:1473-81. [Crossref] [PubMed]
- Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 2007;297:267-77. [Crossref] [PubMed]
- Neoptolemos JP, Palmer D, Ghaneh P, et al. ESPAC-4: A multicenter, international, open-label randomized controlled phase III trial of adjuvant combination chemotherapy of gemcitabine (GEM) and capecitabine (CAP) versus monotherapy gemcitabine in patients with resected pancreatic ductal adenocarcinoma. J Clin Oncol 2016;34:LBA4006. [Crossref]
- Wu W, He J, Cameron JL, et al. The impact of postoperative complications on the administration of adjuvant therapy following pancreaticoduodenectomy for adenocarcinoma. Ann Surg Oncol 2014;21:2873-81. [Crossref] [PubMed]
- Mayo SC, Gilson MM, Herman JM, et al. Management of patients with pancreatic adenocarcinoma: national trends in patient selection, operative management, and use of adjuvant therapy. J Am Coll Surg 2012;214:33-45. [Crossref] [PubMed]
- Varadhachary GR, Fleming JB, Crane CH, et al. Phase II study of preoperation mFOLFIRINOX and chemoradiation for high-risk resectable and borderline resectable pancreatic adenocarcinoma. J Clin Oncol 2015;33:362. [Crossref]
- Okada KI, Shimokawa T, Hirono S, et al. Effect of Neoadjuvant Nab-Paclitaxel plus Gemcitabine Therapy on Overall Survival in Patients with Borderline Resectable Pancreatic Cancer: A Prospective Multicenter Phase II Trial (NAC-GA Trial). Oncology 2017;93:343-6. [Crossref] [PubMed]
- Takahashi H, Ohigashi H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 2013;258:1040-50. [Crossref] [PubMed]
- Chuong MD, Springett GM, Freilich JM, et al. Stereotactic body radiation therapy for locally advanced and borderline resectable pancreatic cancer is effective and well tolerated. Int J Radiat Oncol Biol Phys 2013;86:516-22. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs. Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]
- Colbert LE, Moningi S, Chadha A, et al. Dose escalation with an IMRT technique in 15 to 28 fractions is better tolerated than standard doses of 3DCRT for LAPC. Adv Radiat Oncol 2017;2:403-15. [Crossref] [PubMed]
- Crane CH. Hypofractionated Radiation Therapy With BED >100 Gy May Rival Surgical Outcomes. Int J Radiat Oncol Biol Phys 2017;99:301. [Crossref] [PubMed]
- Ferrone CR. After Neoadjuvant Therapy, Imaging No Longer Provides a Clear Answer. Int J Radiat Oncol Biol Phys 2017;99:300. [Crossref] [PubMed]
- Cassinotto C, Sa-Cunha A, Trillaud H. Radiological evaluation of response to neoadjuvant treatment in pancreatic cancer. Diagn Interv Imaging 2016;97:1225-32. [Crossref] [PubMed]
- Ferrone CR, Marchegiani G, Hong TS, et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann Surg 2015;261:12-7. [Crossref] [PubMed]
- Katz MH, Fleming JB, Bhosale P, et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 2012;118:5749-56. [Crossref] [PubMed]
- Chang JS, Choi SH, Lee Y, et al. Clinical usefulness of (1)(8)F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 2014;90:126-33. [Crossref] [PubMed]
- Choi M, Heilbrun LK, Venkatramanamoorthy R, et al. Using 18F-fluorodeoxyglucose positron emission tomography to monitor clinical outcomes in patients treated with neoadjuvant chemo-radiotherapy for locally advanced pancreatic cancer. Am J Clin Oncol 2010;33:257-61. [PubMed]
- Wilson JM, Mukherjee S, Brunner TB, et al. Correlation of (18)F-Fluorodeoxyglucose Positron Emission Tomography Parameters with Patterns of Disease Progression in Locally Advanced Pancreatic Cancer after Definitive Chemoradiotherapy. Clin Oncol (R Coll Radiol) 2017;29:370-7. [Crossref] [PubMed]
- Lee JH, Kang CM, Bang SM, et al. The Role of Neoadjuvant Chemoradiation Therapy in Patients With Borderline Resectable Pancreatic Cancer With Isolated Venous Vascular Involvement. Medicine (Baltimore) 2015;94:e1233. [Crossref] [PubMed]
- Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg 2018;268:215-22. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Mukherjee S, Hurt CN, Bridgewater J, et al. Gemcitabine-based or capecitabine-based chemoradiotherapy for locally advanced pancreatic cancer (SCALOP): a multicentre, randomised, phase 2 trial. Lancet Oncol 2013;14:317-26. [Crossref] [PubMed]
- Krishnan S, Chadha AS, Suh Y, et al. Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 2016;94:755-65. [Crossref] [PubMed]
- Faris JE, Blaszkowsky LS, McDermott S, et al. FOLFIRINOX in locally advanced pancreatic cancer: the Massachusetts General Hospital Cancer Center experience. Oncologist 2013;18:543-8. [Crossref] [PubMed]
- Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266-74. [Crossref] [PubMed]
- Kim G. nab-Paclitaxel for the treatment of pancreatic cancer. Cancer Manag Res 2017;9:85-96. [Crossref] [PubMed]


