Stop hedging your bets: reasons for non-adherence to a tri-modality regimen in the treatment of esophageal cancer in a multidisciplinary setting
Introduction
Outcomes for patients treated for esophageal cancer have improved in the current era of multi-modality therapy. However, nearly 17,000 Americans will be diagnosed with esophageal cancer in 2018, and a nearly equivalent number will succumb to the disease (1). For locally-advanced esophageal cancer (LAEC), tri-modality therapy (TMT) consisting of chemoradiation (CRT) and esophagectomy remains the standard-of-care in North America (2,3). However, many radiation oncologists have concerns regarding eventual surgical candidacy following neoadjuvant therapy, potentially influencing radiation dose decision making. Consequently, some recommend a modified CROSS regimen (4) consisting of 50 Gy or higher definitive radiation doses with concurrent carboplatin/paclitaxel rather than risking the possibility of no surgery taking place for a patient who had received only a “neoadjuvant” radiation dose (41.4 Gy) per the published CROSS regimen (3,5).
Outside of prospective randomized trials, the course of post-neoadjuvant therapy for patients with LAEC has not been reported. Such information would be of considerable benefit to better aide radiation oncologist behavior in determining an appropriate dose prescription. To this end, we aimed to examine the rate of TMT and reasons for non-adherence to TMT in patients treated at a large multidisciplinary esophageal program.
Methods
From 2007 to 2016, we identified LAEC patients diagnosed from a prospective institutional database. Patients indicated for TMT (documentation of planned TMT at the outset in either tumor board or consultation notes) were divided into CRT/S+ (documentation of completed surgery) and CRT/S- (no subsequent surgery) groups. Detailed chart review provided TMT non-adherence reasons.
Results
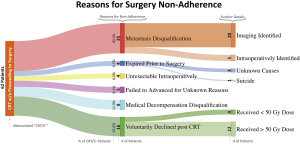
We identified 283 patients with documentation of planned TMT prior to CRT. Of the TMT-indicated patients, 221 (78.1%) completed surgery after CRT (CRT/S+), while 62 (21.9%) failed to advance to surgery (CRT/S-) (Figure 1). A total of 164 (58.0%) patients received 50 or 50.4 Gy CRT, greater than 50.4 Gy for 27 (9.5%), and less than 50 Gy for 92 (32.5%, only 8 patients received RT to 41.4 Gy). Concurrent chemotherapy largely consisted of cisplatin/5FU (predominating pre-CROSS trial therapy) or carboplatin/paclitaxel (post-CROSS trial therapy).
Twenty-five of 62 CRT/S- patients (40.3%) had evidence of metastatic progression following CRT (20 identified on imaging, 5 identified intraoperatively), 4 (6.5%) were unresectable intraoperatively, 4 (6.5%) expired prior to planned surgery (3 from unknown causes, 1 committed suicide), 8 (12.9%) experienced significant medical decompensation from CRT and were no longer surgical candidates, 16 (25.8%) voluntarily declined surgery following CRT (largely due to the concerns of long-term quality of life) and 5 (8.1%) failed to advance to surgery for unknown reasons (Figure 1). Four of the 16 patients who voluntarily declined surgery after CRT received RT doses of less than 50 Gy.
CRT/S+ patients demonstrated a 21.8% pathologic complete response rate (20.3% for adenocarcinoma and 29.0% for squamous cell carcinoma). In total, 157 (90.8%) of 173 patients without pathologic complete responses had no tumor at the resection margins (R0).
Discussion
Our institution’s 78% surgery completion rate among TMT patients highlights the benefits of upfront multi-disciplinary care (6). In addition, we are increasingly utilizing the published CROSS regimen RT doses for surgical candidates. We present every esophageal cancer patient seen by our providers, regardless of stage, at our bi-weekly multidisciplinary Esophageal Care Conference in a prospective manner. Our upper foregut surgical team uses a consistent methodology regarding surgical candidacy. Creating clarity between oncologists, our group is almost always aware of a patient’s eventual surgical candidacy prior to initiating neoadjuvant therapy. This essential multidisciplinary approach allows for the prescription of shorter and more tolerable neoadjuvant regimens.
Our rate of completion of TMT was lower than that of the randomized CROSS trial, where 161 of 171 (94.2%) patients who completed CRT eventually underwent resection. However, the CROSS protocol did not require re-staging imaging, whereas our practice does so routinely per National Comprehensive Cancer Network guidelines. We identified 25 patients in our cohort with metastatic disease immediately prior to surgery. This supports the use of lower pre-operative radiation doses as higher definitive doses will have no bearing on disease outcome for this unfortunate subset of patients.
Possibly, the only patients who may have benefited from definitive doses of radiation in our cohort were those who medically decompensated following CRT thereby negating any further surgical interventions or those who voluntarily declined surgery after CRT, representing 24 out of 283 patients (8.5%). Additionally, only 2.8% (8 of 283) failed to advance to surgery due to documented medical decompensation. Of these 8 patients, four received RT doses of 50 Gy or higher. It remains unknown whether non-definitive doses (<50 Gy) would’ve mitigated the medical decompensation to further allow surgical resection.
Furthermore, despite early engagement of the surgery team, 16 (6%) patients declined esophagectomy voluntarily following CRT. Most cited quality of life concerns. Four of these patients potentially received under-treatment due to delivery of neoadjuvant doses of radiation. These patients possibly felt overwhelmed shortly after diagnosis and only fully processed the ramification of TMT during the course of neoadjuvant therapy. We have not formally assessed whether patients harbor decision regret. To further provide early assistance, patient support groups can provide social and cultural support, assisting patients in surgical decision making prior to neoadjuvant therapy initiation. At our institution, CP, a retired general surgeon, patient advocate, and longtime survivor of TMT for LAEC attends all multidisciplinary tumor conferences. This person makes himself available both in-person and by-phone to patients and caregivers alike who embark on TMT. Frequently, patients appreciate the presence of someone who underwent their journey and now lives a fruitful and normal life.
Minimizing overall radiation dose delivered to the thorax during neoadjuvant CRT undoubtedly reduces toxicity, as mean lung radiation doses have been strongly associated with pulmonary complications post-operatively (7) and increased cardiac radiation doses may portend to ischemia (8), pericardial effusions (9) and resultant detriment to overall survival (10). Surveying 274 radiation oncologists, the majority of respondents believed that 50.4 Gy would yield a higher pathologic complete response (pCR) rate (236, 86%) and increased R0 resection rates (185, 68%) despite greater toxicity (147, 54%) than 41.4 Gy (5). In our study, CRT/S+ patients who received doses of 50 Gy or greater did not see quantitative improvements of pCR and R0 rates compared to the published CROSS trial. This finding is in line with a negative esophageal RT dose-escalation study (11) and a large National Cancer Database analysis which found no survival benefit to neoadjuvant RT above the CROSS regimen (12). Strengths of this present analysis include the large sample size and comprehensive assessment of reasons of failing to advance to surgery. This study is limited by the relatively small number of patients receiving the published CROSS regimen, interfering with statistical comparisons of dose prescriptions.
In summary, a 78% surgery completion rate among TMT patients highlights the benefits of upfront multidisciplinary care. As nearly 25% of our CRT/S- patients declined esophagectomy voluntarily, thorough surgical counseling, patient advocacy, and patient education prior to CRT is essential to avoid under-treatment. For our CRT/S+ patients, pCR and R0 resection rates did not quantitatively improve over the published CROSS trial. In the absence of a demonstration of superiority of radiation doses greater than 41.4 Gy, the robust CROSS regimen should be the standard of care in managing esophageal TMT patients, especially if evaluated upfront in a multidisciplinary setting.
Acknowledgements
None.
Footnote
Conflicts of Interest: Previous presentation: digital poster presentation at ASTRO Annual Meeting 2018 in San Antonio, TX, USA.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Nabavizadeh N, Shukla R, Elliott DA, et al. Preoperative carboplatin and paclitaxel-based chemoradiotherapy for esophageal carcinoma: results of a modified CROSS regimen utilizing radiation doses greater than 41.4 Gy. Dis Esophagus 2016;29:614-20. [Crossref] [PubMed]
- Elliott DA, Rana SR, Nabavizadeh N, et al. Underutilization of the CROSS Regimen Among US Radiation Oncologists: A National Survey of Practice Patterns. Anticancer Res 2018;38:6375-9. [Crossref] [PubMed]
- Jobe BA, Enestvedt CK, Thomas CR Jr. Disease-specific multidisciplinary care: a natural progression in the management of esophageal cancer. Dis Esophagus 2006;19:417-8. [Crossref] [PubMed]
- Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885-91. [Crossref] [PubMed]
- Gayed IW, Liu HH, Yusuf SW, et al. The prevalence of myocardial ischemia after concurrent chemoradiation therapy as detected by gated myocardial perfusion imaging in patients with esophageal cancer. J Nucl Med 2006;47:1756-62. [PubMed]
- Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 2008;70:707-14. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Buckstein M, Rhome R, Ru M, Moshier E. Neoadjuvant chemoradiation radiation dose levels for surgically resectable esophageal cancer: predictors of use and outcomes. Dis Esophagus 2018. [Crossref] [PubMed]