
Combined conventional transarterial chemoembolization with Mitomycin and percutaneous ablation for unresectable hepatocellular carcinoma
Introduction
Conventional transarterial chemoembolization (c-TACE) has been the standard treatment for intermediate stage hepatocellular carcinoma (HCC) and it is associated with increased overall survival (OS) as shown by two meta-analyses (1,2). For very early or early stage HCC, percutaneous ablation is considered a curative option along with surgical resection (3). Currently, there is growing evidence to support combining both loco-regional therapies to improve tumor response and OS (4-6).
Benefits of TACE followed by percutaneous ablation are based on the synergistic effect between those two methods. It results from changes in intra-tumoral hemodynamic characteristics and tumoral cell chemo-sensitivity. An early study from Rossi et al. reported that occlusion of arterial supply with sudden hemodynamic changes in intra-tumoral blood flow leads to lower tissue impedance and increased heat conductivity (7). Goldberg et al. postulated that the zone of coagulative necrosis during thermal ablation can be enlarged by administering doxorubicin, which would promote necrosis of areas under sub-lethal thermal damage, a mechanism that may involve free-radicals (8).
Early application of this combined approach was described by Veltri et al. who treated patients with unresectable non-early HCC and obtained OS rate of 89.7% at 12 months and 67.1% at 24 months (9). A more recent publication also showed benefit in patients with early stage HCC, who presented lower local recurrence when compared to TACE or PTA alone [hazard ratio (HR) =0.309, 95% CI: 0.130 to 0.736, P=0.008 and HR =0.352, 95% CI: 0.158 to 0.787, P=0.011, respectively] (10).
The goal of this study is to retrospectively review our patient population who underwent c-TACE with Mitomycin followed by percutaneous ablation and to determine efficacy and safety of this combined approach, in addition to possible predictive factors for OS and tumor response.
Methods
After Institutional Review Board (IRB) approval (Pro00061244), retrospective analysis was performed identifying patients that underwent c-TACE with Mitomycin followed by percutaneous ablation from 2011 to 2016. The need for informed consent was waived by the IRB. Diagnosis of HCC was established according to European Association for the Study of the Liver (EASL) criteria. Patients with other primary malignancies were excluded. Data collection included age, sex, diabetes, hypertension, coronary artery disease, smoking history, cause of cirrhosis, Child-Pugh classification, Model of End-Stage Liver Disease (MELD) score, Eastern Cooperative Oncology Group (ECOG) status and number of treatments. Laboratory data included alpha-fetoprotein (AFP), albumin, bilirubin and complete blood cell count, in addition to platelet-to-lymphocyte (P/L) ratio, neutrophil-to-lymphocyte (N/L) ratio, and albumin-to-bilirubin (A/B) ratio. Imaging data included number and size of lesions, portal vein invasion and metastatic lesions. Patients were staged according to the Barcelona Clinic Liver Classification (BCLC).
Conventional TACE
c-TACE was performed either through femoral or radial access under moderate sedation provided with Midazolam and Fentanyl. Sub-segmental or segmental treatments were performed whenever possible. If not, lobar infusion was achieved. All embolizations were performed through a 2.8-Fr micro-catheter (Progret® Terumo, Somerset, NJ). A 10-mL solution containing an empiric dose of 10 mg of Mitomycin was combined with 5 mL of ethiodized oil (Lipiodol® Guerbet, Villepinte, Fr), 10 mL of iodine contrast media (Visipaque® GE Healthcare, Waukesha, WI) and 10 mL of normal saline. This solution was distributed in two 30-mL syringes and mixed via a 3-way stopcock until a homogenous solution was obtained (10–15 cycles). The total volume was equally divided in two different containers and one vial of polyvinyl alcohol (PVA) particles 300–500 µm (Cook, Bloomington, IN) was added to one of them. The mixture without PVA was infused first until saturation of tumoral vasculature with ethiodized oil was observed. This was followed by the infusion of the solution containing the embolic particles (PVA). The endpoint was no further tumor vascular enhancement and complete flow stasis within the feeding vessels. Patients were admitted for overnight observation. The number of c-TACE sessions was determined at the discretion of the interventionalist and performed 6–8 weeks apart. The average number of c-TACE sessions was 3/patient.
Percutaneous ablation
Percutaneous ablation was performed with radiofrequency (RF) energy under general anesthesia and conventional CT-guidance (Somatom® 64 Siemens, NJ, USA) 7 days later. First unenhanced CT scan (100 KV, automated mAs modulation, 512×512 pixels, 5 mm slice thickness) of the abdomen was performed for targeting. Up to three Cooltip® (Medtronic) electrodes (15–20 cm in length; 3 cm active tip; 17 G) were inserted into each lesion according to their size. For lesions ≤3 cm a single electrode was utilized, for lesions between 3–5 cm two electrodes were placed and lesions ≥5 cm were treated with 3 electrodes. Adjustments in electrode positions were made based on limited CT scans until correct positioning was obtained in order to achieve 5–10 mm of ablative margin. No CT-fluoroscopy was utilized. Fixed 12 minutes ablation cycle was performed, with automated energy delivery accordant to impedance level. When more than one electrode was used simultaneous ablation was performed via “switching” mode. Final CT scan was obtained after electrode(s) removal to rule out immediate complications.
The average number of sessions was 1.5/patient and the interval between the c-TACE and PTA varied among providers according to their preference, from within the first 24 hours after c-TACE to a few weeks (median: 21 days; range, 1–35 days). Decision to perform RF ablation was defined prior to initial c-TACE in some patients or was included in the treatment strategy after inadequate response following c-TACE.
Efficacy and safety
Efficacy was assessed by OS, time to progression (TTP) and tumor response according to mRECIST criteria on cross-sectional images. Initial cross-sectional imaging was obtained 1 month after each treatment session. Once the complete response was achieved, follow-up images were obtained every 3 months for the first 2 years. An independent radiologist analyzed the images. Percentage of ethiodized oil uptake was determined at the 30-day follow-up contrasted abdominal computed tomography (CT).
Safety was assessed according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Multiple linear regression was conducted to predict the OS and number of progression-free survival days based on age, A/B ratio, P/L ratio, N/L ratio, MELD, ECOG status, number of lesions, mean size of lesions.
Results
Fifty patients were identified (9 females; 41 males) with unresectable HCC who underwent c-TACE followed by RF ablation from 2011 to 2016. Mean age was 63.1 years old (range, 44–84 years old). Mean MELD score was 9.3. Mean lesion size was 4.1 cm (range, 1.3–16.2 cm) (Table 1).
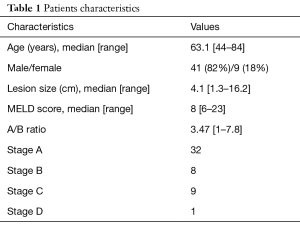
Full table
On follow-up imaging 1 month after treatment, 29 patients (58%) had complete response, 15 patients (30%) had partial response, and 6 patients (12%) had progression of disease. Objective response (CR + PR) was achieved in 44 patients (88%). Images were not available in one patient (Table 2).
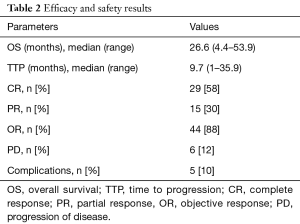
Full table
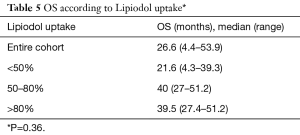
The median OS was 26.6 months (range, 4.4–53.9 months) and median TTP was 9.7 months (range, 1–35.9 months) for the entire cohort. Median OS was also analyzed according to lesion size and results are presented in Table 3. Median OS were also determined according to patient’s BCLC stage. Early (A), intermediate (B), advanced (C) and terminal (D) stages presented the following OS in months: 22.4 (range, 4.3–53.9); 52.5 (range, 51.2–53.8), 25.9 (range, 16–34.5) and 8.8, respectively (Table 4). Finally, median OS in months according to percentage of Lipiodol uptake was as follows (P=0.36): <50%=21.6 (range, 4.3–39.3); between 50–80%=40 (range, 27–51.2); >80%=39.5 (range, 27.4–51.2) (Table 5). There was no statistically significant difference in median OS between patients with different lesion size (P=0.95), different BCLC stage (P=0.84) or different ethiodized oil uptake (P=0.36).
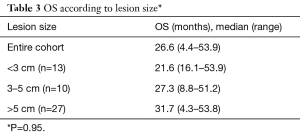
Full table
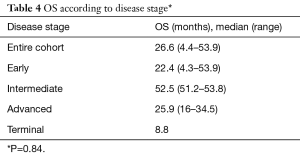
Full table
Full table
Five patients experienced one complication each (5/50, 10%), as follow: two grade 1 (bradycardia), two grade 2 (seizure; pneumothorax) and one grade 4 (acute bleeding). There were no deaths or liver dysfunction related to the procedures. Multiple linear regression showed that higher albumin/bilirubin ratio was significantly correlated with improved OS (P=0.024).
Discussion
Patients with unresectable HCC compose a very heterogeneous population given the wide variability of major prognostic factors, including underlying liver function, tumor burden and portal vein invasion (11). Recent review stated median OS over 60 months for early stage, 26 months (range, 14–45 months) for intermediate stage and 11 (range, 6–20) months for advanced stage (11).
Our results showed a median OS of 26.6 months (range, 4.4–53.9 months) and median TTP of 9.7 months (range, 1–35.9 months) for the entire cohort, which includes patients in different disease stages. Similar to our results, Si et al. reported 66 patients submitted to combined therapy reaching median OS of 21 months and TTP of 9 months (12). Also, Zhang et al. showed a median OS of 29 months in 45 patients beyond Milan criteria that had TACE followed by PTA (13). In addition, there was a statistically significant correlation between higher albumin/bilirubin ratio and improved OS (P=0.024). This is likely related to overall better liver function of this subgroup of patients.
When OS was analyzed according to BLCL stage, the following results were obtained: early-stage, 22.4 months; intermediate-stage, 52.5 months; advanced-stage, 25.9 months and advanced, 8.8 months. Although not statistically significant (P=0.84), OS for was higher for intermediate stage, showing potential benefit in this subgroup.
Radiological response is a predictor of survival after local-regional therapy for hepatic tumors as shown by Memon et al. who demonstrated that patients who responded to the treatment based on the European Association for the Study of the Liver (EASL) criteria had longer survival than non-responders (P=0.0001) (14). In this study, complete response was achieved in 58% and 30% of the patients had partial response, providing an objective response of 88%. This is higher compared to the 52.5% of objective response reported with c-TACE only in a recent systematic review including over 10,000 patients (15). In addition, retrospective review comparing both approaches showed disease progression in 19.29% of patients submitted to TACE only and 3.88% in patients submitted to combined therapy with P=0.015 (16).
Similarly, ethiodized oil accumulation within the tumor has been shown to be a predictor of tumor necrosis as demonstrated by Takayasu et al., who compared CT findings with resected specimens. In a total of 41 tumors good correlation (r=83) was found between retained Lipiodol and necrotic tissue under pathological analysis (17). Although not statistically significant in this population, more likely due to small sample size, a patient with higher Lipiodol uptake experienced longer OS [<50%=21.6 months (range, 4.3–39.3 months) vs. 39.5 months (range, 27.4–51.2 months) with >80% uptake; P=0.36].
Both loco-regional therapies have excellent safety profiles with very low incidence of major complications. Overall, intra-arterial therapy has less than a 5% rate of major complications, including liver dysfunction and post-embolization syndrome requiring hospital admission (18). Percutaneous ablation has reported overall complication rate of 7%, hemorrhage being the most frequent one (19). Different from what was believed initially, combined therapy does not imply a higher complication rate as demonstrated by meta-analysis with no statistical significant difference in major complications (odd ratio =1.20, 95%CI: 0.31–4.62, P=0.79) (6). Our cohort had 5 complications (10%) total, most were minor (only one major complication, acute bleeding), and there were no treatment related deaths.
This study has limitations, such as the small sample size what may have prevented more statistically significant results. In addition, there was a lack of standardized protocol among the providers, leading to great variability in the number of c-TACE sessions and the interval between the intra-arterial therapy and RF ablation, which could had been less than 24 hours to several weeks. Finally, for the c-TACE procedure oil-in-water emulsion (1:6) was utilized and not the opposite. Water-in-oil emulsion was shown to have significantly higher carriage capacity and longer release time for the chemotherapeutic agent (20,21).
Conclusions
In conclusion, this retrospective review showed that combination of c-TACE and RF ablation is a safe and effective approach for patients with unresectable HCC. Albumin/bilirubin ratio was a predictor for improved OS.
Acknowledgments
Funding: The study was sponsored by Guerbet.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2019.01.07). RY and MG report grants from Guerbet, during the conduct of the study; other from Guerbet, outside the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was approved by Institutional Review Board (IRB) of Medical University of South Carolina (Pro00061244) and the need for informed consent was waived by the IRB.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology 2002;224:47-54. [Crossref] [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [Crossref] [PubMed]
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020-2. [Crossref] [PubMed]
- Chen QW, Ying HF, Gao S, et al. Radiofrequency ablation plus chemoembolization versus radiofrequency ablation alone for hepatocellular carcinoma: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2016;40:309-14. [Crossref] [PubMed]
- Lan T, Chang L, Rahmathullah MN, et al. Comparative Efficacy of Interventional Therapies for Early-stage Hepatocellular Carcinoma: A PRISMA-compliant Systematic Review and Network Meta-analysis. Medicine 2016;95:e3185. [Crossref] [PubMed]
- Ni JY, Liu SS, Xu LF, et al. Meta-analysis of radiofrequency ablation in combination with transarterial chemoembolization for hepatocellular carcinoma. World J Gastroenterol 2013;19:3872-82. [Crossref] [PubMed]
- Rossi S, Garbagnati F, Lencioni R, et al. Percutaneous radio-frequency thermal ablation of nonresectable hepatocellular carcinoma after occlusion of tumor blood supply. Radiology 2000;217:119-26. [Crossref] [PubMed]
- Goldberg SN, Kamel IR, Kruskal JB, et al. Radiofrequency ablation of hepatic tumors: increased tumor destruction with adjuvant liposomal doxorubicin therapy. AJR Am J Roentgenol 2002;179:93-101. [Crossref] [PubMed]
- Veltri A, Moretto P, Doriguzzi A, et al. Radiofrequency thermal ablation (RFA) after transarterial chemoembolization (TACE) as a combined therapy for unresectable non-early hepatocellular carcinoma (HCC). Eur Radiol 2006;16:661-9. [Crossref] [PubMed]
- Song MJ, Bae SH, Lee JS, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med 2016;31:242-52. [Crossref] [PubMed]
- Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [Crossref] [PubMed]
- Si ZM, Wang GZ, Qian S, et al. Combination Therapies in the Management of Large (≥5 cm) Hepatocellular Carcinoma: Microwave Ablation Immediately Followed by Transarterial Chemoembolization. J Vasc Interv Radiol 2016;27:1577-83. [Crossref] [PubMed]
- Zhang L, Yin X, Gan YH, et al. Radiofrequency ablation following first-line transarterial chemoembolization for patients with unresectable hepatocellular carcinoma beyond the Milan criteria. BMC Gastroenterol 2014;14:11. [Crossref] [PubMed]
- Memon K, Kulik L, Lewandowski RJ, et al. Radiographic response to locoregional therapy in hepatocellular carcinoma predicts patient survival times. Gastroenterology 2011;141:526-35, 535.e1-2.
- Lencioni R, de Baere T, Soulen MC, et al. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology 2016;64:106-16. [Crossref] [PubMed]
- Dhanasekaran R, Khanna V, Kooby DA, et al. Chemoembolization Combined with RFA for HCC: Survival Benefits and Tumor Treatment Response. J Cancer Ther 2013;4:493-9. [Crossref]
- Takayasu K, Arii S, Matsuo N, et al. Comparison of CT findings with resected specimens after chemoembolization with iodized oil for hepatocellular carcinoma. AJR Am J Roentgenol 2000;175:699-704. [Crossref] [PubMed]
- Brown DB, Nikolic B, Covey AM, et al. Quality improvement guidelines for transhepatic arterial chemoembolization, embolization, and chemotherapeutic infusion for hepatic malignancy. J Vasc Interv Radiol 2012;23:287-94. [Crossref] [PubMed]
- Gervais DA, Goldberg SN, Brown DB, et al. Society of Interventional Radiology position statement on percutaneous radiofrequency ablation for the treatment of liver tumors. J Vasc Interv Radiol 2009;20:3-8. [Crossref] [PubMed]
- Kan Z, Wright K, Wallace S. Ethiodized oil emulsions in hepatic microcirculation: in vivo microscopy in animal models. Acad Radiol 1997;4:275-82. [Crossref] [PubMed]
- de Baere T, Zhang X, Aubert B, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology 1996;201:731-5. [Crossref] [PubMed]


