The role of sequential radiation following adjuvant chemotherapy in resected pancreatic cancer
Introduction
Adjuvant therapy for resected pancreatic cancer remains one of the most highly debated topics in oncology. For patients with resectable disease that have undergone upfront resection, without neoadjuvant therapy, there exists numerous treatment options (1). These include chemotherapy (CT) alone, CT followed by chemoradiotherapy (CRT), and CRT between separate courses of CT. This lack of consensus largely stems from the conflicting results of seminal prospective trials (2-5), as well as the lack of a completed phase III trial to date regarding the direct utility of radiotherapy (RT) in the adjuvant setting.
Two large retrospective institutional analyses have observed survival benefits to adjuvant CRT over observation (6-8). However, the survival impact of RT in patients receiving adjuvant CT remains unclear. A randomized phase II study demonstrated no OS differences between adjuvant CT and CRT, but the trial was not powered sufficiently (9). For this reason, the Radiation Therapy Oncology Group (RTOG) launched the 0848 phase III trial to evaluate 5–6 adjuvant cycles of gemcitabine (plus or minus erlotinib) with or without 5-FU or capecitabine-based CRT.
In the absence of completed randomized trial results, adjuvant management for resected pancreatic cancer remains a contentious topic. Addressing this knowledge gap, this study queries a large cohort with long-term follow-up of this clinically challenging issue, evaluating differences in outcomes between CT with or without sequential RT.
Methods
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society that consists of information regarding tumor characteristics, patient demographics, and patient survival for approximately 70% of the United States population (10). The NCDB contains information not included in the Surveillance, Epidemiology, and End Results database, including details regarding use of systemic therapy. The data used in this study were derived from a de-identified NCDB file. The American College of Surgeons and the CoC have not verified and are neither responsible for the analytic or statistical methodology employed nor the conclusions drawn from these data. As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation. The project was reviewed by our local institutional review board (COMIRB) and deemed to be exempt due to deidentified status of the data.
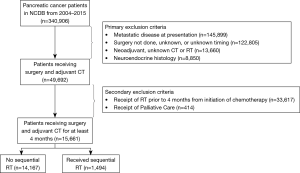
The NCDB Participant User File corresponding to pancreatic cancer [2004–2015] was utilized for this investigation. Inclusion criteria were nonmetastatic, histologically-confirmed pancreatic carcinoma status post oncologic resection with adjuvant CT. Additional exclusion criteria were receipt of neoadjuvant therapy, unknown CT and/or RT status, intraoperative CT and/or RT, as well as non-external beam RT modalities. Any patients coded as receiving palliative care were excluded. Patients with unknown timing of CT, RT, and/or surgery were excluded, as it could not be ascertained whether they received therapy pre- or post-operatively. Lastly, in order to emulate RTOG 0848, patients were removed if they initiated RT less than 4 months following the start of CT (Figure 1).
In accordance with the variables in NCDB files, information collected on each patient broadly included demographic, clinical, and treatment data. All variables were selected a priori. Demographic variables including age, year of diagnosis, race/ethnicity, insurance status, Charlson-Deyo (CD) comorbidity score, distance from facility, facility type, facility location, and median income were defined according to their respective data fields in the NCDB data dictionary (11). Race was categorized as non-Hispanic White, Hispanic, Black, or other. Facility regions were grouped into Northeast, South, Midwest, and West regions. Insurance status was grouped into government (Medicare/Medicaid/other), private, or uninsured. Comorbidity was defined as per the CD comorbidity score (12). Distance from facility was grouped at ≤10 or >10 miles straight-line distance. Income was coded based on census tract estimates and are not patient-specific.
For pathologic factors, pathological tumor (pT) and nodal (pN) stage per the 7th edition of AJCC staging were collected. Other factors included margin status, pre-treatment carbohydrate antigen (CA) 19-9, defined as either ≤90 or >90 U/mL, and location of primary defined as head of pancreas or other. The treatment factor evaluated, as all patients received adjuvant CT, was whether they also received radiation, at a minimum of 4 months from initiation of chemotherapy. A 4-month time point was chosen to allow for completion of at least 4 out of 5 cycles of monthly chemotherapy required per the RTOG 0848 protocol.
Statistical analysis was performed with SPSS statistical software (version 24.0; IBM Corporation, Armonk, NY). Tests were two-sided, with a threshold of P<0.05 for statistical significance. Clinical characteristics of the overall cohort were tabulated and inter-group comparisons were made with the chi-squared test. Multivariable logistic regression analysis was performed to ascertain factors independently associated with receipt of sequential RT and propensity scores were generated. Median follow-up was calculated using the reverse Kaplan-Meier method.
Survival analyses were performed using the log-rank test for univariate analysis (UVA) and Cox proportional hazards regression for multivariate analysis (MVA) to estimate hazard ratios. Initial variable selection included all variables discussed previously. The final parsimonious multivariate Cox model was formed by using multivariate hierarchical backwards selection of variables at a significance level of P<0.10. The proportional hazards assumption was assessed for all variables in the final multivariate analysis and was not violated (13). For propensity adjustment, propensity score was manually entered into the model to determine any changes in determinants of survival.
Results
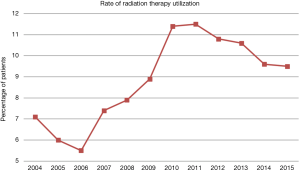
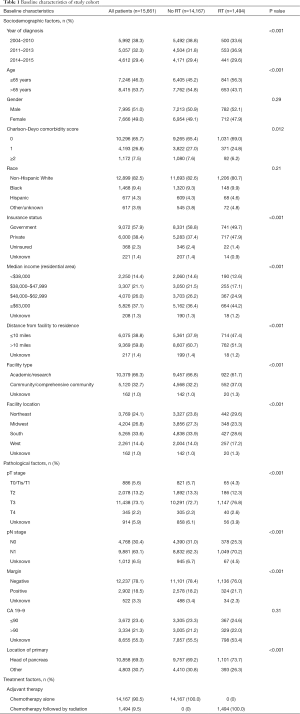
A patient selection diagram is illustrated in Figure 1. Of 15,661 patients, 14,167 (90.5%) underwent CT alone, and 1,494 (9.5%) received RT (Table 1). The majority of cases were pT3, node-positive, margin negative, with primary disease located in the pancreatic head. Utilization of RT was at its lowest in 2006, at 5.5% of patients, and peaked in 2011 and 11.5%, before gradually decreasing to 9.5% in 2015 (Figure 2).
Full table
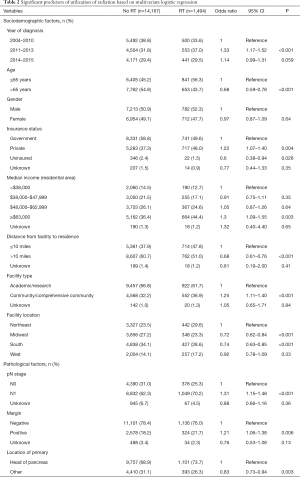
Multivariable logistic regression analysis was performed to evaluate independent predictors of receiving CT and RT as compared to CT alone (Table 2). These patients were more often younger, had higher income, had private insurance, lived closer to treating facilities, treated at community centers, and were diagnosed at more recent time periods (P<0.05 for all). In addition to sociodemographical differences, RT patients also tended to have a pancreatic head primary, node positive disease, and margin positive resection (P<0.05 for all).
Full table
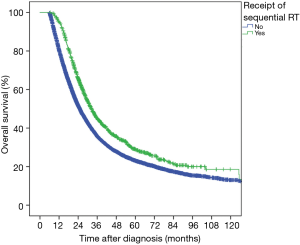
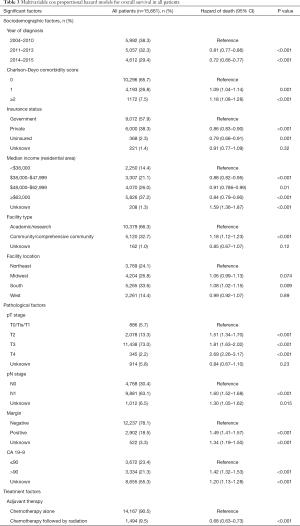
At a median follow-up of 53.6 months, 3-year OS in the entire cohort was 36.3%. On UVA, patients receiving sequential RT had improved OS compared to adjuvant CT alone (3-year OS 44.6% vs. 35.3%, P<0.001, Figure 3). On MVA, multiple sociodemographic factors that improved OS included more recent year of diagnosis, CD score of 0, non-governmental insurance, higher income residential area, treatment at academic facility, and treatment in the Northeast (P<0.05, Table 3). Pathologic factors that worsened OS included increasing pT stage, nodal positivity, positive margin, and CA 19-9 values >90 U/mL (P<0.05, Table 3). The benefit of sequential RT after CT was maintained on MVA (HR =0.68; 95% CI, 0.63–0.73; P<0.001, Table 3). After propensity score adjustment, the benefit of RT was maintained (HR =0.68; P<0.001).
Full table
Discussion
In the absence of randomized data evaluating adjuvant CT with or without sequential RT for resected pancreatic cancer, this investigation of a large, contemporary national database addresses a major knowledge gap for this highly debated issue. Sequential RT after adjuvant CT in resected pancreatic cancer improves survival in this patient cohort. While this is the point of study per the ongoing RTOG 0848 trial, clinicians do not have guidance at the current time for ideal treatment. Utilization of RT over time has varied depending on year of diagnosis, with initially low numbers at a nadir of 5.5% of patients, likely related to publication of the ESPAC-1 trial (2,3). There is an increase in utilization around when RTOG 0848 was announced and initiated accrual. after 2006, followed by a plateau of usage around 10% as oncologists await results of RTOG 0848.
In this patient cohort, RT was delivered to subjects at higher risk for locoregional failure, including those with node positive disease and positive margins. Addition of adjuvant RT is more accepted in patients with more advanced primary disease, high-grade tumors, nodal disease, high CA 19-9, and/or positive surgical margins (14). Many of these parameters were associated with a higher likelihood of RT delivery in the current analysis. Despite these poorer prognostic factors, the fact that sequential RT remained associated with higher OS on UVA is noteworthy. However, patients receiving RT were more likely to be younger and treated more recently with potentially better chemotherapy regimens. Lastly, allowing for four months of adjuvant CT followed by RT selects for those patients who are less likely to fail systemically, therefore potentially benefiting more from aggressive local therapy, leading to the OS benefit observed.
The value of additional adjuvant RT in pancreatic cancer follows a general principle, namely that patients at high risk for locoregional recurrence and low risk for metastatic recurrence are most likely to benefit. It is difficult to evaluate the relative risks of locoregional versus distant recurrence in pancreatic cancer, given the inherently high metastatic potential. Although ample patterns-of-failure studies exist, they are often confounded by the use of antiquated CT regimens (15,16). This shortcoming is a substantially important one, because improved systemic control for this disease would likely shift failure patterns locoregionally. With evidence of improved outcomes with more aggressive CT regimens, such as FOLFIRINOX (17) or gemcitabine and capecitabine (18), preventing locoregional failure may become more important. Subgroup analysis of RTOG 0848 will be crucial to evaluate which patients benefit most from RT, although most of these analyses will be post-hoc and may not be adequately powered.
The Gastrointestinal Tumor Study Group (GITSG) 9173 trial was a prematurely terminated study that randomized 43 patients status post R0 resection to observation versus adjuvant 5-fluorouracil (5-FU)-based CRT followed by maintenance 5-FU; there was a statistical improvement in OS with the latter (4). A similar design to GITSG 9173 was applied in the European Organisation for the Research and Treatment of Cancer (EORTC) 40891 study, which showed a nonsignificant trend (P=0.099) towards OS benefit in the CRT arm (5). The European Study Group for Pancreatic Cancer (ESPAC)-1 study randomized patients in a 2×2 factorial design to +/− adjuvant CT and +/− sequential CRT (2,3). That study demonstrated no statistically apparent OS benefit for RT patients, with a potential survival detriment associated with RT.
Although GITSG 9173 demonstrated a benefit to CRT, and EORTC 40891 and ESPAC-1 did not, several issues have severely limited applicability of any of those trials. RT delivered in each trial was delivered with antiquated techniques, at low doses, and/or using ineffective fractionation schemes, such as split course. EORTC 40891 was criticized for not stratifying by surgical margin status along with the lack of maintenance CT. Some have also pointed out that using a one-sided log-rank test between arms would have achieved statistical significance (19). Flaws in ESPAC-1 have also been well-characterized, including treating physicians’ ability to choose the randomization group, utilization of physician-chosen prior therapy regardless of randomization arm, and lack of central audit (20).
Lessons learned from the LAP-07 trial in unresected disease may be extrapolated to the adjuvant setting (21). In this phase III trial, CRT showed significant improvements in local control, but the competing risk of distant progression was too large in patients with unresected disease. If systemic control could be achieved more frequently by better source control (through surgery) and more active chemotherapy regimens, locoregional control becomes more important. Because unresected cases have a much higher risk of distant failure, and thus worse OS, than resected cases, the value of local therapy in resected patients may be proportionally greater. The value of OS as the primary endpoint of phase III trials evaluating a local modality for a disease that is most likely to recur distantly may overshadow conclusions.
The primary limitation of this study is that the sequential RT patients may have been a very well-selected cohort owing to the sequential receipt of RT following at least 4 months of CT. Patients who progressed within those 4 months would have been less likely to receive RT and thus would not have been included in the RT cohort; however, it may be that the best role for adjuvant RT for resected patients should be for those who have been treated with systemic treatment first, only receiving RT for those who do not progress. This is important because the current analysis does not maintain that criteria. We do not know what percentage of patients in this study progressed prior to completion of their planned adjuvant treatment. A portion of the CT only patients may have developed metastatic disease while on chemotherapy, thus never receiving RT.
In addition to the lack of information on tumor response to CT as mentioned above, NCDB studies have several noteworthy shortcomings. In addition to retrospective selection biases, the NCDB does not carry well-coded information on specific CT agents/cycles, performance status, RT target volumes, and salvage therapies, all which could affect OS and confound conclusions of the current study. It also does not record other endpoints, such as tolerance of therapy, locoregional failure, and cancer-specific survival. While patients confirmed to have received palliative care were excluded, that variable is not well coded enough to strongly suggest that all other patients did not receive any palliative therapy. Next, because the NCDB only gives relative intervals from the start of a particular therapy, it was not possible to evaluate whether the CRT cohort received CT followed by CRT, or CT followed by RT alone. It also cannot assess additional CT delivered following CRT, which is an accepted management option (1). Lastly, although the NCDB includes data for 70% of the United States population, only CoC-accredited facilities contribute data; as such, these findings may not necessarily be representative of the entire United States population.
Conclusions
In the absence of randomized data evaluating adjuvant CT with or without sequential RT or CRT for resected pancreatic cancer, this investigation shows that sequential RT appears to improve OS in well-selected patients who have received 4 months of adjuvant chemotherapy. These data merit consideration in fervent anticipation of definitive results from publication of RTOG 0848.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: As all patient information in the NCDB database is de-identified, this study was exempt from institutional review board evaluation. The project was reviewed by our local institutional review board (COMIRB) and deemed to be exempt due to deidentified status of the data.
References
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma. Clinical Practice Guidelines in Oncology, version 1, 2016. Available online: https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf
- Neoptolemos JP, Stocken DD, Friess H, et al. A Randomized Trial of Chemoradiotherapy and Chemotherapy after Resection of Pancreatic Cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. [Crossref] [PubMed]
- Kalser MH, Ellenberg SS. Pancreatic cancer. Adjuvant combined radiation and chemotherapy following curative resection. Arch Surg 1985;120:899-903. [Crossref] [PubMed]
- Klinkenbijl JH, Jeekel J, Sahmoud T, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg 1999;230:776-82; discussion 782-4. [Crossref] [PubMed]
- Herman JM, Swartz MJ, Hsu CC, et al. Analysis of Fluorouracil-Based Adjuvant Chemotherapy and Radiation After Pancreaticoduodenectomy for Ductal Adenocarcinoma of the Pancreas: Results of a Large, Prospectively Collected Database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. [Crossref] [PubMed]
- Corsini MM, Miller RC, Haddock MG, et al. Adjuvant Radiotherapy and Chemotherapy for Pancreatic Carcinoma: The Mayo Clinic Experience (1975-2005). J Clin Oncol 2008;26:3511-6. [Crossref] [PubMed]
- Hsu CC, Herman JM, Corsini MM, et al. Adjuvant Chemoradiation for Pancreatic Adenocarcinoma: The Johns Hopkins Hospital—Mayo Clinic Collaborative Study. Ann Surg Oncol 2010;17:981-90. [Crossref] [PubMed]
- Van Laethem JL, Hammel P, Mornex F, et al. Adjuvant Gemcitabine Alone Versus Gemcitabine-Based Chemoradiotherapy After Curative Resection for Pancreatic Cancer: A Randomized EORTC-40013-22012/FFCD-9203/GERCOR Phase II Study. J Clin Oncol 2010;28:4450-6. [Crossref] [PubMed]
- Bilimoria KY, Stewart AK, Winchester DP, et al. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol 2008;15:683-90. [Crossref] [PubMed]
- American College of Surgeons. National Cancer Data Base: Participant user file data dictionary. Version: PUF 2016 – Containing cases diagnosed in 2004-2016. Available online: https://www.facs.org/~/media/files/quality%20programs/cancer/ncdb/puf_data_dictionary.ashx
- Quan H, Li B, Couris CM, et al. Updating and Validating the Charlson Comorbidity Index and Score for Risk Adjustment in Hospital Discharge Abstracts Using Data From 6 Countries. Am J Epidemiol 2011;173:676-82. [Crossref] [PubMed]
- Bellera CA, MacGrogan G, Debled M, et al. Variables with time-varying effects and the Cox model: Some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol 2010;10:20. [Crossref] [PubMed]
- Chuong MD, Boggs DH, Patel KN, et al. Adjuvant chemoradiation for pancreatic cancer: what does the evidence tell us? J Gastrointest Oncol 2014;5:166-77. [PubMed]
- Asiyanbola B, Gleisner A, Herman JM, et al. Determining Pattern of Recurrence Following Pancreaticoduodenectomy and Adjuvant 5-Flurouracil-Based Chemoradiation Therapy: Effect of Number of Metastatic Lymph Nodes and Lymph Node Ratio. J Gastrointest Surg 2009;13:752-9. [Crossref] [PubMed]
- Winter JM, Tang LH, Klimstra DS, et al. Failure Patterns in Resected Pancreas Adenocarcinoma. Ann Surg 2013;258:331-5. [Crossref] [PubMed]
- Conroy T, Hammel P, Hebbar M, et al. Unicancer GI PRODIGE 24/CCTG PA.6 trial: A multicenter international randomized phase III trial of adjuvant mFOLFIRINOX versus gemcitabine (gem) in patients with resected pancreatic ductal adenocarcinomas. J Clin Oncol 2018;36:LBA4001. [Crossref]
- Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. [Crossref] [PubMed]
- Garofalo MC, Regine WF, Tan MT. On Statistical Reanalysis, the EORTC Trial Is a Positive Trial for Adjuvant Chemoradiation in Pancreatic Cancer. Ann Surg 2006;244:332-3. [Crossref] [PubMed]
- Koshy MC, Landry JC, Cavanaugh SX, et al. A challenge to the therapeutic nihilism of ESPAC-1. Int J Radiat Oncol Biol Phys 2005;61:965-6. [Crossref] [PubMed]
- Hammel P, Huguet F, van Laethem JL, et al. Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 2016;315:1844-53. [Crossref] [PubMed]