
Mucinous adenocarcinoma arising from chronic perianal fistula—a multidisciplinary approach
Introduction
Perianal fistula (PF) is a common entity in the proctology area. However, the development of a mucinous adenocarcinoma (MA) arising from chronic anal fistula is extremely rare. The relation between both entities was first described by Rosser et al. in 1934, who reported seven cases of fistula that had undergone malignant transformation (1). The pathogenesis of this malignancy is still controversial. MA has an indolent growth and, although metastases to inguinal lymph nodes may be present in advanced cases, distant metastases are uncommon (2-4). However, these locally aggressive neoplasms entail a high probability of local recurrence. The subtle symptoms that resemble a fistula-associated perianal MA to an inflammatory benign pathology make an early diagnosis difficult (5).
There is no uniform consensus until date regarding diagnosis and/or therapeutic strategies due to the lack of randomized prospective trials embracing this rare malignancy. Multimodality therapies for locally advanced anorectal cancer are currently well recognized treatment options that improve the outcomes. Pre- and postoperative combined chemoradiation therapy (CRT), in association with surgery and wide resection margins is, up until now, the best available alternative, achieving better survival rates (4,6,7).
Unfortunately, as the diagnosis is often delayed, some of these patients have locally advanced cancer at the time of surgery, which results in poor prognosis (3).
Due to the lack of available data and definitive therapeutic guidelines, the aim of this study is to present our experience based on the outcomes after combining preoperative concurrent chemoradiotherapy, ischioanal APR and eventual adjuvant chemotherapy, and discuss the clinical features with respect to safety and efficacy of the multimodality therapy.
Case presentation
Patient A
A 66-year-old male, without medical records, complained about pain and perianal oozing for one month. The patient referred a growth in the perianal region with no history of any altered bowel habits. Physical examination revealed multiple deformities of the perianal region, with surrounding induration and the presence of several external fistula openings (Figure 1A).
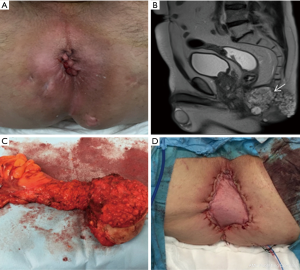
Anorectal digital examination evidenced a stony and fixed to deep planes extramucosal mass, occupying the half posterior of the inferior rectum and anal canal. Colonoscopy confirmed the integrity of the rectal mucosa, and histological examination of the biopsy taken from the fistula tract showed epithelial cells and well differentiated dilated tortuous glands with lakes of mucin, consistent with the diagnosis of MA. Pelvic MRI showed a large mass with high signal intensity in T2 sequency extending to both ischioanal fossas, without any pelvic or inguinal lymph nodal involvement (Figure 1B). Thoraco-abdominal computed tomography scan (CT) did not reveal distant metastases.
Given these findings, management options were discussed by a multidisciplinary team, and the patient was proposed to undergo preoperative chemoradiation followed by surgery. Neoadjuvant treatment consisted in chemotherapy with capecitabine, and concurrent 3-dimensional conformal radiotherapy to a dose of 50.4 Gy in 28 fractions. Post-CRT re-staging MRI did not show a decrease in the tumor size. Twelve weeks after preoperative CRT, the patient underwent an ischioanal APR, and a VRAM flap was used for the perineal reconstruction (Figure 1C,D). Histological findings confirmed the mucinous nature, with only 3% of malignant cellularity and wide free-tumor margins. The perineo-gluteal wound healed completely and he was discharged on postoperative day 16.
Subsequently, the patient completed the treatment with adjuvant oral capecitabine for four months. No evidence of local recurrence or distant metastases has been observed after 28 months of follow-up.
Patient B
A 71-year-old male with a 10-year history of complex anal fistula for which he had undergone a fistulotomy and three previous drainages, complained about persistence of symptoms and a recurrent posterior horseshoe-shaped abscess. Physical examination revealed chronic inflammatory perineal tissue, with two external openings found at both buttocks. Colonoscopy showed no abnormality. Histological examination of the biopsy performed revealed a low grade signet-ring cell MA (Figure 2). Pelvic MRI evidenced a 95 mm length tumor present in the pelvis, extending from the posterior wall of the lower third of the rectum to the external anal margin (Figure 2A,B). Contrast-enhanced thoraco-abdominal CT scan revealed multiple inguinal lymph nodes, with no evidence of distant metastases.
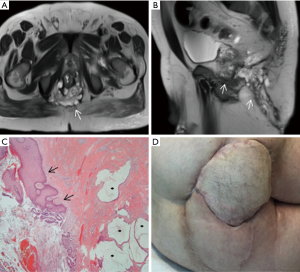
Based on these findings, the patient was first subjected to neoadjuvant chemoradiotherapy. Post-CRT re-staging MRI examination on the fifth week showed a moderate response of the tumor (grade 3). Ten weeks later, the patient underwent an ischioanal APR, and perineal reconstruction was made using a right VRAM flap. Also, a left fasciocutaneous flap was advanced into the wound defect for a better covering (Figure 2D). Histopathological examination of the specimen confirmed a low-grade MA with few malignant cellularity, adequate tumor-free margins and the absence of lymph nodal involvement. Unfortunately, the patient developed a viral pneumonia that prolonged his length of stay for 66 days. No other complications were observed, and the patient was discharged in good condition and with suitable healing of the perineum. Because of the extended length of stay, the patient did not receive adjuvant chemotherapy.
The patient had remained free from disease for 26 months follow-up. At that time, an abdominal CT scan revealed an enlarged left inguinal lymph node, which malignancy was confirmed on biopsy; yet no local recurrence has been observed until date.
Patient C
A 62-year-old male presented with history of painful defecation and bleeding per rectum, in association with mucinous perianal drainage for 3 years. He gave a past history of complex anal fistula for which he had undergone an excision of the fistula tract and two additionally abscess drainages. Inspection of perineal region revealed an indurated area with several external anal fistula openings with spontaneous discharge of mucinous material. Colonoscopy showed circumferential ulceration of the mucosa in the lower anal canal, and a 2 cm length infiltrative tumor just proximal to the anal verge. Histological examination of a biopsy taken from one of the fistula tracts confirmed infiltration by a well differentiated MA (Figure 3). Pelvic MRI revealed a large demarcated mucinous lesion, measuring 49 mm × 42 mm × 71 mm. The tumor occupied the left elevator muscle of the anus with cranial extension to the left mesorectal fat (Figure 3A). Preoperative contrast-enhanced CT scan evidenced one enlarged left external iliac lymph node. No distant metastases were observed. The treatment outcome with preoperative chemoradiation therapy followed by ischioanal APR, nine weeks later. Post-CRT re-staging MRI examination did not show any evidence of tumor regression. A VRAM flap was used for perineal reconstruction (Figure 3D). Pathological response was evaluated using the tumor regression grading system criteria of the Japanese Society for the Cancer of Colon and Rectum (8), confirming the absence of primary tumor and a complete response (G0) after preoperative chemoradiotherapy (ypT0N0).
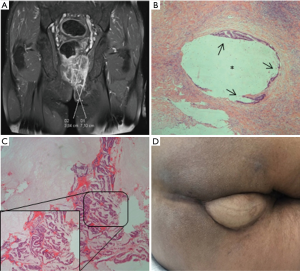
Postoperative course was uneventful. The patient had a suitable wound healing, and was discharged on postoperative day 14. He received postoperative chemotherapy with oral capecitabin for four months. No recurrences or distant metastases have been observed after 19 months of follow-up.
Discussion
According to the World Health Organization (WHO), MA is an invasive adenocarcinoma consisting of malignant glandular cells which contain intracytoplasmic mucin. Usually, the infiltrating glandular structures are associated with mucoid stromal formation (9).
MA can appear not only in multiple locations of the digestive tract (colon, stomach, pancreas, gallbladder, etc.), but also in other placements like breast, thyroid, or even skin. However, perianal location of MA is very uncommon. It has been suggested that malignant degeneration of a long-standing PF is often associated with mucosal regeneration, while other authors believe malignant cells settle in the fistulous granulation tissue arising from proximal gastrointestinal neoplasms (10). Repeated friction, scarring and inflammatory reactions may be predisposing risk factors for development of perianal MA (5,11). The association of Crohn’s disease with MA has been widely established, and a high suspicion has to be maintained in the presence of perianal fistulas (12-14).
Symptoms at presentation usually include perianal pain, itching, mucinous discharge and/or abscess, in association with an ulcero-proliferative growth or palpable mass in the perianal region (3). Considering the unusual infiltration of rectal mucosa, neither intestinal obstruction nor rectal bleeding are very common symptoms (5,15,16). The most frequent clinical presentation is related to long-standing fistula-in-ano (1); tumor progression may imply tissue destruction, resulting in perianal abscesses and anal fistulas, with the consequent development of symptoms as perianal oozing and/or mucinous discharge (6).
Early diagnosis of perianal adenocarcinoma is difficult as the tumor does not pierce the rectal mucosa and its course is usually indolent. Therefore, clinical suspicion is of paramount importance in the diagnosis, as the symptoms often mimic benign inflammatory conditions. In our series, patients’ symptoms were attributed to their perianal benign diseases, and neither previous biopsy samples nor clinical suspicion of malignant carcinoma were performed. Thus, further colorectal investigation was not achieved, and an early diagnosis was missed.
Digital rectal examination evidences a thickened indurated area involving the fistula tract (6), while colonoscopic examination shows no growth in anorectum. Imaging modalities as CT scan, MRI and endoscopic ultrasound (EUS) establish the disease extension to adjacent tissues and help to plan the surgical strategy (7-11). Among them, pelvic MRI is the best image technique, since the abundance of mucin in these tumors gives them a unique radiological appearance, which results in a significant hyper-intense signal on T2-weighted images (17). However, MRI images can be difficult to distinguish from other fluid-containing pathologies such as cysts, fluid collections, and even necrotic tumors. The presence of a fistula tract involved by the mass and the anal canal is a representative characteristic of MA over PF (18). Functional imaging technique like PET-CT is not a valid evaluation tool, since the presence of mucina may result in false negatives due to poor 2-[fluorine-18]-fluoro-2-deoxy-D-glucose (FDG) uptake (19).
Histological diagnosis remains the gold standard. The presence of extracellular mucinous lakes surrounded by well differentiated dilated tortuous glands, nerves and vessels, confirms the diagnosis. In many cases, definitive diagnosis may be verified by the histopathological examination of the resected specimen because preoperative biopsies can fail to reveal an infiltrating carcinoma (2,20).
Surgery is considered the cornerstone in the management of this malignant entity. Most surgeons support the APR with wide local excision as the preferred therapeutic option, as it helps in removing the malignant tissue, while reducing the chances of local recurrence when negative margins are achieved (21). In locally advanced tumors, either an ischioanal APR or extralevator abdominoperineal excision (ELAPSE) is needed in order to decrease positive margins rates. In these specific situations, the reconstruction of the pelvic floor is required, meaning an increased operative time and a higher morbidity rate, too.
However, there is a high level of uncertainty surrounding the combination of chemo- and radiotherapy, either in preoperative or adjuvant setting, still unresolved. Yang et al. suggested that the combination of chemoradiotherapy is a valid and adequate option when the tumor is not completely resected or the patient refuses surgery (5). Also, Hongo et al., in an 11-patient study, reported more disease-free survival in those cases where chemoradiotherapy was given prior to surgery (22). In a retrospective study including 82 patients, Belkacémy et al. conclude that the stage T and N, the histological grade, and the therapeutical modality are independent risk factors for survival rate, achieving better outcomes with chemoradiotherapy, and recommended surgery as a salvage approach (23). Hence, in locally advanced tumors, preoperative chemoradiotherapy causes downsizing of large neoplasms, contribute to eliminate disseminated tumor cells, thus increases the chances of R0 resection and decreases the incidence of local recurrences (21).
Besides that, it has been reported that mucinous status observed at pretherapeutic MRI was associated with a noticeably worse response to chemoradiation than non-mucinous tumors in rectal cancer (24).
In view of the extensive involvement of surrounding soft tissues and the histopathological reports evidenced in our cases, upfront surgery was not considered due to the possibility of a R2 resection. We particularly chose to treat our patients with preoperative chemoradiotherapy, followed by aggressive surgery such as ischioanal APR, and subsequently adjuvant chemotherapy. Also, post-CRT re-staging MRI did not show a significant tumor regression in any of our patients; however, far fewer active tumor cells were evidenced in the histopathological examination of the specimen, even though there was not a downstaging based on the tumor size.
Distant metastases are rare in MA, and tumor spread is usually lymphatic, being the inguinal nodes the most frequent site of metastases (21). According to the published data, prognosis seems to be worse when the tumor is larger than 5 cm size, carcinoembryonic antigen is elevated, or lymph nodal or haematogenous metastases are present at the time of diagnosis (25,26), finding reported survival rates of 2–48 months (27).
Long-term survival rates are not yet satisfactory if the patient has locally advanced stage disease.
Conclusions
Mucinous adenocarcinoma arising from chronic perianal fistula is an uncommon neoplasm with indolent growth and locoregional aggressiveness. As these tumors have a high risk of local recurrence, an aggressive and multimodal approach should be carried out to reduce positive surgical margin rates and increase R0 resection rates.
Despite the limited follow-up and the small sample size in the present series, we believe that combined chemoradiotherapy, followed by an aggressive surgery with ischioanal APR and adjuvant chemotherapy, are appropriate in locally advanced tumors, without major increased morbidity. However, further experience is required to establish the optimal management and accurate treatment in these uncommon malignancies.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Informed Consent: Written informed consent was obtained from all individual participants included in the study for publication of this case report and any accompanying images.
References
- Rosser C. The relation of fistula in ano to cancer of the anal canal. Trans Am Proctol Soc 1934;35:65-71.
- Okada K, Shatari T, Sasaki T, et al. Is histopathological evidence really essential for making a surgical decision about mucinous carcinoma arising in a perianal fistula? Report of a case. Surg Today 2008;38:555-8. [Crossref] [PubMed]
- Inoue Y, Kawamoto A, Okigami M, et al. Multimodality therapy in fistula-associated perianal mucinous adenocarcinoma. Am Surg 2013;79:e286-8. [PubMed]
- Gaertner WB, Hagerman GF, Finne CO, et al. Fistula-associated anal adenocarcinoma: good results with aggressive therapy. Dis Colon Rectum 2008;51:1061-7. [Crossref] [PubMed]
- Yang BL, Shao WJ, Sun GD, et al. Perianal mucinous adenocarcinoma arising from chronic anorectal fistulae: a review from single institution. Int J Colorectal Dis 2009;24:1001-6. [Crossref] [PubMed]
- Sierra EM, Villanueva Saenz E, Martínez PH, et al. Mucinous adenocarcinoma associated with fistula in ano: report of a case. Tech Coloproctol 2006;10:51-3. [Crossref] [PubMed]
- Ibáñez Aguirre FJ, Erro Azcárate JM, Aranda Lozano F, et al. Mucinous adenocarcinoma on chronic perianal fistula treated by neoadjuvant QT-RT and laparoscopic abdomino-perineal resection. Rev Esp Enferm Dig 2006;98:310-2. [Crossref] [PubMed]
- Japanese Society for Cancer of the Colon and Rectum. General Rules for Clinical and Pathological Studies on Cancer of the Colon, Rectum and Anus. Kanehara & CO., LTD. Tokyo. 2005.
- Fritz A, Percy C, Jack A, et al. International Classification of Diseases for Oncology. Third edition. Geneva: World Health Organization. 2000.
- Dukes CE, Galvin C. Colloid carcinoma arising within fistulae in the anorectal region. Ann R Coll Surg Engl 1956;18:246-61. [PubMed]
- Purkayastha A, Sharma N, Dutta V, et al. Mucinous adenocarcinoma of perianal region: an uncommon disease treated with neo-adjuvant chemo-radiation. Transl Gastroenterol Hepatol 2016;1:52. [Crossref] [PubMed]
- Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn's disease. Colorectal Dis 2003;5:490-5. [Crossref] [PubMed]
- Ky A, Sohn N, Weinstein MA, et al. Carcinoma arising in anorectal fistulas of Crohn's disease. Dis Colon Rectum 1998;41:992-6. [Crossref] [PubMed]
- Church JM, Weakley FL, Fazio VW, et al. The relationship between fistulas in Crohn’s disease and associated carcinoma: report of four cases and review of the literature. Dis Colon Rectum 1985;28:361-6. [Crossref] [PubMed]
- Ohta R, Sekikawa K, Goto M, et al. A case of perianal mucinous adenocarcinoma arising from an anorectal fistula successfully resected after preoperative radiotherapy. Case Rep Gastroenterol 2013;7:219-23. [Crossref] [PubMed]
- Papapolychroniadis C, Kaimakis D, Giannoulis K, et al. A case of mucinous adenocarcinoma arising in long-standing multiple perianal and presacral fistulas. Tech Coloproctol 2004;8:s138-40. [Crossref] [PubMed]
- Chand M, Yu S, Swift RI, et al. Mucinous carcinoma of the rectum: a distinct clinicopathological entity. Tech Coloproctol 2014;18:335-44. [Crossref] [PubMed]
- Ho CM, Tan CH, Ho BC. Clinics in diagnostic imaging (143). Perianal mucinous adenocarcinoma arising from chronic fistula-in-ano. Singapore Med J 2012;53:843-8. [PubMed]
- Mistrangelo M, Lesca A. PET-CT in Anal Cancer: Indications and Limits. In: Misciagna S, editor. Positron Emission Tomography - Recent Developments in Instrumentation, Research and Clinical Oncological Practice. InTech, 2013:235-56.
- Baars JE, Kuipers EJ, Dijkstra G, et al. Malignant transformation of perianal and enterocutaneous fistulas is rare: results of 17 years of follow-up from the Netherlands. Scand J Gastroenterol 2011;46:319-25. [Crossref] [PubMed]
- Santos MD, Noqueira C, Lopes C. Mucinous Adenocarcinoma Arising in Chronic Perianal Fistula: Good Results with Neoadjuvant Chemoradiotherapy Followed by Surgery. Case Rep Surg 2014;2014:386150. [Crossref] [PubMed]
- Hongo K, Kazama S, Sunami E, et al. Perianal adenocarcinoma associated with anal fistula: a report of 11 cases in a single institution focusing on treatment and literature review. Hepatogastroenterology 2013;60:720-6. [PubMed]
- Belkacémi Y, Berger C, Poortmans P, et al. Management of primary anal canal adenocarcinoma: a large retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys 2003;56:1274-83. [Crossref] [PubMed]
- Oberholzer K, Menig M, Kreft A, et al. Rectal cancer: mucinous carcinoma on magnetic resonance imaging indicates poor response to neoadjuvant chemoradiation. Int J Radiat Oncol Biol Phys 2012;82:842-8. [Crossref] [PubMed]
- Prasad SN, Razik A, Siddiqui F, et al. Mucinous adenocarcinoma arising from chronic perianal fistula mimicking horseshoe abscess. BMJ Case Rep 2018. [Crossref] [PubMed]
- Marti L, Nussbaumer P, Breitbach T, et al. Perianal mucinous, adenocarcinoma. A further reason for histological study of anal fistula or anorectal abscess. Chirurg 2001;72:573-7. [Crossref] [PubMed]
- Anthony T, Simmang C, EL, Lee EL, et al. Perianal mucinous adenocarcinoma. J Surg Oncol 1997;64:218-21. [Crossref] [PubMed]


