The downstaging approach to irresectable oesophageal and gastric cancer: a single centre experience
Introduction
In the United Kingdom (UK) oesophageal and gastric (OG) adenocarcinomas present as stage III or IV disease in 70–80% of cases (1). In the 20–30% of patients who present with potentially resectable disease the standard of treatment is neoadjuvant chemotherapy, reassessment of disease, followed by surgical resection and consideration of adjuvant chemotherapy depending on pathology results. This approach is supported by several large datasets (2-4) which demonstrate a survival advantage compared to surgical resection alone.
Where patients present with advanced tumours which are irresectable at time of radiological diagnosis due to local disease burden, but with no evidence of distant metastatic disease outside a conventional resection field, there is uncertainty as to the optimal therapeutic strategy. Historically, many patients would have been managed palliatively from time of diagnosis. However, there is evidence that some patients respond well to chemotherapy to the point of clinical stage decreasing (5), i.e., the local disease burden has reduced to the point that they may become candidates for surgical resection. In conjunction with the establishment of robust neoadjuvant treatment pathways, this had led to the emergence of a downstaging strategy. In this setting, cases which are conventionally irresectable can be managed with an initial radical intent, pending adequate response to preoperative chemotherapy. Conversely, there are also patients who progress despite chemotherapy, who fail to respond, or who develop toxicity to treatment and become unfit for surgery.
To date there are no currently published data describing the outcomes following single modality (chemotherapy) downstaging management of irresectable OG cancers. A greater understanding of outcomes in these cases is needed to guide management. The identification of patient and pathological factors which influence both prognosis and chance of progression to resection will allow tailoring of therapy and more effective delivery of care.
This study aims to describe the experiences of a regional service in treating patient with initially irresectable OG cancers with a downstaging chemotherapy approach and identify survival and morbidity outcomes following resection.
Methods
Data collection
This retrospective study identified cases which were discussed at regional MDT over a 32-month period between 01/01/15 and 09/08/17. Data were collected from electronic patient records, ChemoCare, MDT databases, and MDT records. Treatment intent in eligible cases in our service is recorded as “downstaging” and this was used as a search parameter to identify cases.
Eligibility
In our regional service downstaging management follows a similar pathway to neoadjuvant care, detailed in Figure 1. Additional investigations such as endoscopic ultrasound (EUS) and magnetic resonance imaging (MRI) may be considered on a case-by-case basis. Patients who respond to chemotherapy to the extent that their disease becomes resectable proceed to a trial of dissection.
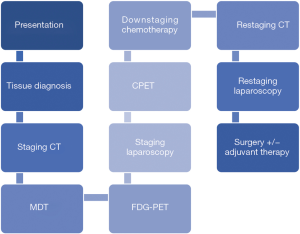
Cases considered for downstaging management must have biopsy-proven gastric or oesophageal adenocarcinoma, with no evidence of distant metastatic disease following protocolised staging, and locally advanced disease including (but not limited to): fixed nodal mass at laparoscopy, lesser curve fat infiltration, coeliac axis lymphadenopathy, T4 disease with local invasion (pleura, crura, pericardium). Patients must also be fit enough to undergo chemotherapy and surgery. Patients are only commenced on downstaging treatment following agreement from all disciplines present (surgery, oncology, radiology, pathology) that they are eligible after MDT discussion.
Exclusion criteria included squamous cell pathology, incomplete datasets, and patients currently undergoing treatment.
Downstaging treatment
Our treatment pathway is summarised in Figure 1. The downstaging component consists of a planned 6 cycles of chemotherapy following diagnosis, with radiological restaging after 3 cycles. After completion of all cycles, additional restaging using computed tomography (CT) and staging laparoscopy is carried out. Equivocal cases may undergo computed tomography-positron emission tomography (CT-PET) following MDT review. Treatment cessation may occur after 3 cycles if there is radiological evidence of progression or a lack of any response; this must be agreed by all members of MDT. First line chemotherapy is epirubicin, cisplatin and capecitabine/5-fluorouracil (ECX/ECF).
Interpretation of any CT/PET imaging was based on reporting by the specialist radiology team attached to our regional MDT. Response to treatment was taken from these radiology reports and categorised as “good”, “partial”, “no response/static”, or “progression of disease”. The Response Evaluation Criteria in Solid Tumours (RECIST) criteria are used as a basis for these categories (6).
Outcomes
Primary outcome was proportion of patients who progressed to resection with clear surgical margins (R0). Secondary outcomes were overall survival (OS), recurrence-free survival (RFS), proportion of patients successfully completing all planned cycles of chemotherapy, chemotherapy induced toxicity, post-operative complications [assessed using Clavien-Dindo grading (7)], 30- and 90-day post-operative mortality and post-operative length of stay. Survival was calculated from date of confirmed tissue diagnosis, recurrence was defined as date of radiological or endoscopic diagnosis of recurrent disease.
Statistics
Data are reported as median [interquartile range (IQR)] unless specified. For non-parametric continuous data, Mann-Whitney-U tests were used for bivariate analyses and Kruskal-Wallis tests for multivariate analyses. Categorical data were analysed using the Chi-squared test. Time to event analyses were calculated by the Kaplan-Meier method with significance shown by the log-rank P value. Statistical analyses were calculated using SAS Studio 3.71 (©SAS Institute Inc., Cary, NC, USA). P values of <0.05 were considered statistically significant.
Statement of ethics approval
As per our local guidelines formal ethics committee approval is not required for this study (study design: retrospectively collected case series). All data were handled in compliance with Caldicott guidelines.
Results
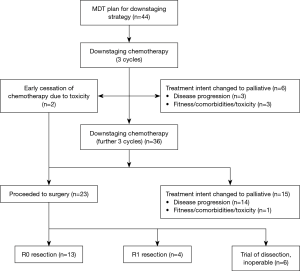
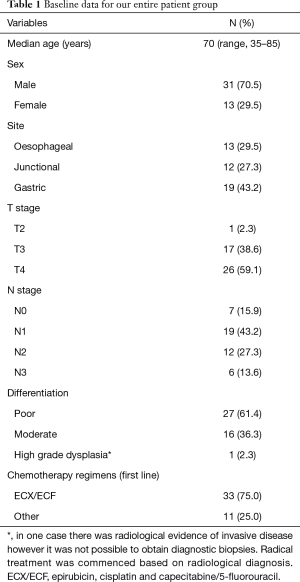
A total of 44 patients were included; baseline demographic and pathological data are shown in Table 1. Patient progression through treatment is shown in Figure 2.
Full table
Chemotherapy toxicity and dropouts
Two patients underwent early trial of dissection due to cessation of chemotherapy due to toxicity; in 1 case this was cardiac-type chest pain (which did not necessitate any cardiology intervention), and in 1 case the patient experienced severe gastrointestinal (GI) toxicity. Three patients stopped chemotherapy prior to completing their planned course due to stroke (n=1), and overall reduced performance status (n=2); in each of these cases treatment intent was changed to palliative due to this toxicity. One patient was declined from surgery following completion of downstaging therapy due to deterioration in fitness from time of diagnosis. Fourteen patients were found to have progressed at restaging after 6 cycles of chemotherapy; 3 of these were detected at laparoscopy and the remaining 11 were radiologically detected.
ECX/ECF were used as first line chemotherapy in 33 patients. One of these patients was changed to a carboplatin-based regime due to acute coronary event during treatment but went on to complete all cycles of chemotherapy. Eight patients had EOX/EOF used first line, 3 patients had carboplatin-based regimes first line due to prior ischaemic heart disease.
Data on response to chemotherapy at first restaging CT were available for 39 patients. Response was “static” in 8 cases, “partial” in 11 cases, “good” in 14 cases, and disease progression was present in 6 cases.
Response to chemotherapy at final restaging CT was recorded in 33 cases; “static” in 11 cases, “partial” in 5 cases, “good” in 11 cases, and disease progression was present in 6 cases. There were no differences in baseline characteristics between any of the categories of response to chemotherapy.
Surgical outcomes
Twenty-three patients completed downstaging chemotherapy and proceeded to trial of dissection. Six of these were found to have inoperable disease at laparotomy and went on to undergo palliative management.
Seventeen patients underwent surgical resection, of which 10 were gastric tumours, 3 were junctional, and 4 were oesophageal. Median time from tissue diagnosis to resection was 8.2 months (range, 5.4–21.6 months). There was no difference in baseline patient or disease characteristics in the group which proceeded to surgery and the group which were changed to palliative management.
Type of resection, complications from surgery, and eventual pathology following surgery are shown in Table 2. Thirteen patients had no evidence of microscopic disease at surgical margin; the remaining 4 were found to have R1 resection. In one case no primary tumour could be identified at pathological examination and the patient was therefore regarded as complete pathological responder. Most common change in T stage from diagnosis to resection was −1.
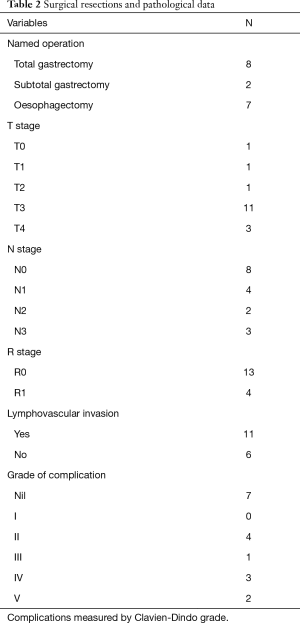
Full table
Complications within 90 days of surgery occurred in 10 of the patients who underwent resection. Two patients died, both due to complications resulting from anastomotic leak. Three developed respiratory failure requiring reintubation, 1 developed a pneumothorax requiring intercostal drain, 1 developed sepsis requiring HDU admission and 3 developed post-operative pneumonia which did not necessitate critical care admission. Post-operative 30-day mortality was 0.0%; 90-day mortality was 11.8%. Median length of stay following resection was 16 days (range, 11–90 days).
OS/RFS
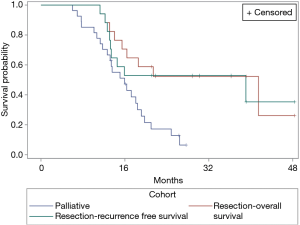
Median follow-up for all patients was 16.8 months, median follow-up for the cohort which proceeded to resection was 22.0 months. Figure 3 shows OS for both the surgical and palliative cohorts, and RFS for the surgical cohort.
Median OS in the cohort which underwent resection was 42.6 months compared with 16.4 months in the cohort undergoing palliative management. Median RFS in the surgical cohort was 40.1 months. Both OS and RFS in the resection group were significantly increased compared to the cohort managed palliatively (P=0.0057).
All patients who had R1 resection margins were diagnosed with recurrence (median time from surgery to recurrence 7.7 months, range, 7.1–30.8 months).
Discussion
Despite improvements in patient education, referral criteria for non-specialists, and the development of regional cancer networks, the majority of cases of upper GI cancers are advanced at time of presentation (1). Broadening the scope of cases which can undergo radical treatment is therefore a key step in the advancement of upper GI cancer management.
In our cohort of patients managed with downstaging treatment 29.5% of patients underwent R0 resection with 38.6% of patients undergoing either R0 or R1 resection. The cohort of patients which underwent surgery had superior survival outcomes to those eventually managed palliatively. Survival in our palliative cohort was similar to other reported series (8). A 90-day postoperative mortality in our resection cohort was higher than other reported series (9,10), and was higher than our own institutional mortality rate for all upper GI cancer resections. This likely reflects our small sample size and the difficulties of operating in patients with significant residual disease burden. Complication rates of both chemotherapy and surgery are broadly similar to those reported elsewhere (9-11). Increased survival in our surgical cohort compared with the palliative cohort may be influenced by disease phenotype; those who progressed to resection may have more chemo-sensitive disease and this may influence OS. It remains unclear if these patients who respond well to chemotherapy would have similar survival outcomes regardless of whether they underwent surgery or not.
The majority of our patients did not proceed to resection due to either a lack of response to chemotherapy or fitness/toxicity issues. Therefore, the development of novel chemotherapy regimens with increased efficacy and/or decreased toxicity may serve to augment the existing downstaging pathway. The FLOT4 study, comparing the docetaxel-based FLOT regime with ECF/ECX, has shown a survival benefit with comparable toxicity profile (12). Human epidermal growth factor receptor 2 (HER2) expression in gastric cancer may be up to 17.9% (13). Trastuzumab (a monoclonal antibody targeted against HER2) has been shown to improve survival when combined with conventional palliative chemotherapy in HER-2 positive tumours in the ToGa trial (14) and is currently being assessed in the neoadjuvant setting by the INNOVATION trial (15). The phase II HER-FLOT study is evaluating the perioperative use of combination FLOT and trastuzumab, and promising early results report high pathological complete response rates (16). None of the variables which we measured at baseline on both patient or disease characteristics had any influence on predicting response to chemotherapy. Prognostic factors predicting both survival and response to chemotherapy could be incorporated into future scoring systems to enhance the selection process into downstaging treatment pathways.
We believe that our data support the use of a downstaging management strategy to convert initially irresectable disease to resectable disease and offer increased numbers of patients a radical treatment option. Further work investigating novel chemotherapy regimens and targeted immunotherapy in the downstaging setting is needed. Future studies may incorporate the identification of factors which predict response to chemotherapy to allow tailored treatment regimens and improved patient selection to the downstaging pathway.
Limitations
The major limitations of our study are small sample size, limited follow-up period, and single region recruitment. Our outcomes did not include functional status or quality of life measures.
Acknowledgements
None.
Footnote
Conflicts of Interest: This article previously presented at AUGIS Annual Meeting 19-21 September 2018.
Ethical Statement: As per our local guidelines formal ethics committee approval is not required for this study (study design: retrospectively collected case series). All data were handled in compliance with Caldicott guidelines.
References
- Cancer Research UK. Oesophageal cancer incidence statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oesophageal-cancer/incidence#ref-3
- Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006;355:11-20. [Crossref] [PubMed]
- Ychou M, Boige V, Pignon JP, et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: an FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011;29:1715-21. [Crossref] [PubMed]
- Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012;379:315-21. [Crossref] [PubMed]
- Davies AR, Gossage JA, Zylstra J, et al. Tumor stage after neoadjuvant chemotherapy determines survival after surgery for adenocarcinoma of the esophagus and esophagogastric junction. J Clin Oncol 2014;32:2983-90. [Crossref] [PubMed]
- Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur J Cancer 2016;62:132-7. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Wagner AD, Unverzagt S, Grothe W, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;17:CD004064. [PubMed]
- Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol 2014;21:3008-14. [Crossref] [PubMed]
- Gockel I, Exner C, Junginger T. Morbidity and mortality after esophagectomy for esophageal carcinoma: a risk analysis. World J Surg Oncol 2005;3:37. [Crossref] [PubMed]
- Geh JI, Glynne-Jones R, Kwok QS, et al. Preoperative ECF chemotherapy in gastro-oesophageal adenocarcinoma. Clin Oncol (R Coll Radiol) 2000;12:182-7. [PubMed]
- Al-Batran SE, Homann N, Schmalenberg H, et al. Perioperative chemotherapy with docetaxel, oxaliplatin, and fluorouracil/leucovorin (FLOT) versus epirubicin, cisplatin, and fluorouracil or capecitabine (ECF/ECX) for resectable gastric or gastroesophageal junction (GEJ) adenocarcinoma (FLOT4-AIO): A multicenter, randomized phase 3 trial. J Clin Oncol 2017;35:abstr 4004.
- Abrahao-Machado LF, Scapulatempo-Neto C. HER2 testing in gastric cancer: An update. World J Gastroenterol 2016;22:4619-25. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Integration of Trastuzumab, with or without Pertuzumab, into Perioperative Chemotherapy of HER2- Positive Stomach Cancer: The INNOVATION Trial (EORTC-1203-GITCG). Oncol Res Treat 2016;39:153-4; discussion 155. [Crossref] [PubMed]
- Hofheinz R, Hegewisch-Becker S, Thuss-Patience PC, et al. HER-FLOT: Trastuzumab in combination with FLOT as perioperative treatment for patients with HER2-positive locally advanced esophagogastric adenocarcinoma: A phase II trial of the AIO Gastric Cancer Study Group. J Clin Oncol 2014;32:abstr 4073.