
Vertebral body irradiation during chemoradiation therapy for esophageal cancer contributes to acute bone marrow toxicity
Introduction
Esophageal cancer is a leading cause of cancer morbidity and mortality, contributing to 442,000 new cases and 440,000 deaths globally in 2013 (1). In the United States, although there were 17,000 reported new cases of esophageal cancer in 2016, the 5-year overall survival (OS) based on national data remains low at 18% (2). Surgery has traditionally been the mainstay for localized esophageal cancer, but it alone results in high locoregional failure rates ranging from 30–60% (3-6).
The central role of radiotherapy in the management of esophageal cancer was established through several seminal trials studies which established trimodality treatment as the standard of care in locally advanced esophageal cancer (7-9). These randomized data showed that neoadjuvant chemoradiation (CRT) doubles 3-year OS rates from 6–16% to 30%. Also pathologic complete responses (pCR) improve from 5–15% with neoadjuvant chemotherapy alone to 20–30% using neoadjuvant chemoradiation (7-9). In 2008, Tepper et al. reported that trimodality therapy resulted in a 5-year survival rate of 40% compared to surgery alone (10). Clinical toxicities of CRT include expected grade ≥3 esophagitis (42%); however, more than half of the patients experienced at least one occurrence of grade ≥3 hematologic toxicity (HT) (57%). HT can result in dose-reductions, interruptions in chemotherapy, or potentially unplanned radiation treatment breaks (11).
While trials such as the CALGB 80803, PRODIGE5, and PROTECT-1402 employ multidrug regimens which may improve they efficacy of treatment, they often do so at the cost of increasing the rates of grade ≥3 HT (12-14). Nevertheless, RT likely also contributes to HT when given in combination with chemotherapy.
We therefore hypothesized that radiation dose to the bone marrow was associated with development of HT in esophageal cancer patients who were treated with carboplatin-paclitaxel based CRT.
Methods
Patient population
After an institutional review board-approval, we retrospectively reviewed a total of 97 patients who were managed with curative intent therapy (definitive CRT or preoperative CRT followed by surgery) from 2005–2015. For purposes of homogeneity and data interpretation, we included only patients who received preoperative carboplatin-paclitaxel and radiation therapy (15,16). Of the 97 patients screened with available weekly complete blood counts (CBC) with differentials during CRT, those who received colony-stimulating factors, a chemotherapy regimen other than carboplatin-paclitaxel, or did not complete a full course of chemoradiation were excluded, leaving 53 patients for consideration.
Treatment planning and delivery
Computed tomography (CT) scan simulation was performed with the patient in supine position with arms up in a wingboard with body immobilization. Vertebral bodies (VB) were contoured from the C2 to L2 vertebra or the most inferior complete vertebra visualized on simulation scans. Each vertebral contour included the body, pedicles, transverse processes, laminae, and the spinous processes. The spinal canal was excluded. The ribs, scapulae, and clavicles were contoured separately. Weekly intravenous carboplatin (area under the curve =2) and paclitaxel (50 mg/m2) was delivered during radiation therapy. Forty-four patients (83%) were treated to a dose of 5,040 cGy with either IMRT or 3D conformal radiation therapy (3DCRT) radiation. Three patients (5.7%) were treated to doses between 4,140–5,040 cGy and 5 patients (9.4%) all of whom had upper esophageal cancer were treated to doses between 5,040–7,000 cGy.
Evaluation
White blood cell (WBC), hemoglobin (Hgb), and platelet (Plt) counts were collected weekly starting from the onset of neoadjuvant chemoradiation, through the duration of treatment, and before each scheduled post-treatment follow-up for 90–120 days after CRT. The Common Terminology Criteria for Adverse Events version 4.0 (CTCAE v4.0) was used to evaluate the grade of HT. Dose volume histogram (DVH) data were collected in percentages of VB V10–V60 (VBV), thoracic rib V10–V60 (TRV), scapula V10–V60, and clavicle V10–V60 in increments of 10. VBV5, TRV5, scapula V5, clavicle V5, mean vertebral dose (MVD), mean rib dose (MRD), mean scapula dose (MSD), and mean clavicle dose (MCD) were collected as well. A DVH parameter of Vx was defined as the percentage of organ receiving at least x Gy of radiation.
Statistical analysis
DVH parameters were obtained for all patients and the Shapiro-Wilks test was performed to assess for normality of hematologic cell values. Non-normally distributed values were log transformed. Univariate and multiple linear regressions were performed to identify associations between WBC, Hgb, and Plt nadirs and DVH parameters. Multiple linear regression models were controlled for body mass index (BMI) and age at diagnosis as previous studies established these factors to be associated with WBC count nadir (17,18). The regression coefficient (β) was estimated and represents the change in mean WBC count for every 1-unit increase in corresponding DVH parameter. Univariate logistic regression was used to assess the risk of grade ≥3 HT with increasing DVH parameters. Receiver operating characteristic (ROC) curves were calculated to determine the dose thresholds for avoiding grade ≥3 HT. These thresholds correspond to the point closest to the upper left portion of the graph, which represents the highest accuracy of predicting HT. Data analyses were performed on SAS v9.3 (SAS Institute, Cary, NC, USA). Statistically significant P values were accepted at a level below 0.05.
Results
Patient characteristics
Patient characteristics are shown in Table 1. Of the 53 patients included with locally advanced esophageal carcinoma, 37.7% (n=20) had squamous cell carcinoma and 62.3% (n=33) had adenocarcinoma. Sixty-six percent (n=35) presented with gastroesophageal (GE) junction cancer, 22.6% (n=12) presented with thoracic esophageal cancer, and 11.3% (n=6) presented with cervical esophageal cancer. The average primary tumor size was 76.1 cc (range, 8.4–231.1 cc) and 67.9% (n=36) were node positive at diagnosis. Mean age at diagnosis was 67 years (range, 34–90 years). There was no statistically significant correlation of leukopenia on linear regression with BMI or logistic regression analysis with gender, location of tumor, and histology.
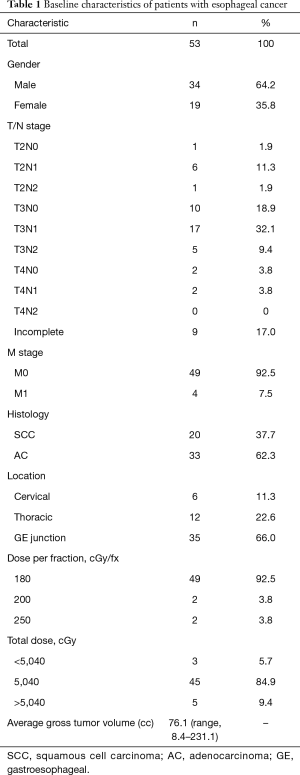
Full table
HT rates
Descriptive characteristics of HT during CRT are shown in Table 2. Mean and median baseline blood count values were WBC of 7.1 and 6.8 k/µL (range, 4.5–12.8 k/µL), Hgb of 12.6 and 13 k/µL (range, 9.2–15.5 k/µL), and Plts of 250.7 and 242.0 k/µL (range, 149–2,358 k/µL). Of the 53 patients included in this study, 5.7% (n=3) did not develop leukopenia, 56.6% (n=30) developed grade 1–2 leukopenia, and 37.7% (n=20) developed grade 3–4 leukopenia. Seventeen percent (n=9) did not develop anemia, while 69.8% (n=37) developed grade 1–2 anemia and 13.2% (n=7) developed grade 3 anemia. A proportion of 18.9% (n=10) did not develop thrombocytopenia, while 71.7% (n=38)developed grade 1–2 thrombocytopenia and 9.4% (n=5) patients developed grade 3 thrombocytopenia (Table 3). No patients developed grade 4 anemia or thrombocytopenia. Patient ANC levels were not available weekly due to the CBC being done without a differential analysis and could not be calculated for 22 of the 53 patients. All patient nadirs occurred within the timeframe of the chemoradiation treatment rather than after completion of CRT.
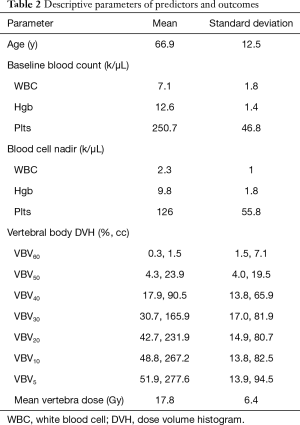
Full table

Full table
Dosimetric parameters associated with HT
On univariate linear regression, VBV30 (β=−0.004; P=0.017), VBV20 (β=−0.005; P=0.006), VBV10 (β=−0.006; P=0.002), VBV5 (β=−0.006; P=0.002), and MVD (β<−0.001; P=0.018) were negatively linearly associated with mean log transformed WBC nadir by percentage. Plots of the WBC nadir with respect to VBV10 are shown in Figure 1.
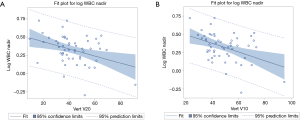
When controlled for BMI and age at diagnosis, multiple linear regression analyses (Table 4) revealed that VBV30 (β=−0.004; P=0.012), VBV20 (β=−0.005; P=0.006), VBV10 (β=−0.006; P=0.002), VBV5 (β=−0.006; P=0.002), and MVD (β=−0.0001; P=0.011) were negatively correlated with mean log transformed WBC nadir by percentage of volume.
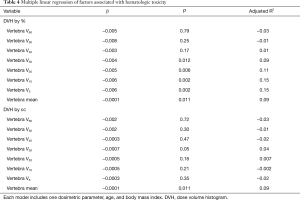
Full table
Irradiation of the ribs, clavicle, and scapula was not significantly associated with leukopenia. Anemia and thrombocytopenia were not associated with increased bone marrow irradiation of any structure. Clinical parameters (BMI, gender, age at diagnosis) and tumor/treatment parameters (GTV volume in cc, total RT dose, and number of fractions) were not associated with WBC nadirs.
Determining thresholds to avoid HT
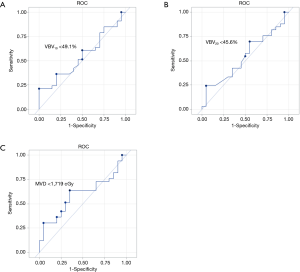
ROC analysis was performed to determine cutoffs to avoid grade ≥3 leukopenia. These DVH cutoffs were V10 <49.1%, VBV20 <45.6%, and MVD <17.2 Gy (Figure 2).
Discussion
Our study demonstrates that increasing low dose and mean radiation dose to the VB is significantly associated with development of leukopenia in esophageal cancer patients receiving CRT, particularly VBV5, VBV10, VBV20, VBV30, and MVD. Cutoffs to avoid grade ≥3 leukopenia were V10 <49.1%, VBV20 <45.6%, and MVD <17.2 Gy. Our study is relevant to patients treated with chemoradiation for esophageal cancer in which radiation total doses were in the definitive range of 5,040 cGy.
Decreasing HT by mitigating bone marrow irradiation in patients undergoing CRT has been incorporated across various cancer sites and with differing chemotherapy regimens. Radiation of the pelvic bone marrow (PBM) in gynecologic and anal cancer patients contributes to WBC and ANC nadirs despite differences in irradiation volumes, treatment dosage, and concurrent chemotherapy (17,19). Mell et al. demonstrated cervical cancer patients with V10 ≥90% had higher rates of grade 2–3 leukopenia (11.1% to 73.7%, P<0.01) and greater risk of discontinuing chemotherapy (OR 32.2; 95% CI, 1.67–622; P=0.02). Similarly, V20 ≥75% resulted in increased grade 2–3 leukopenia (23.8% to 68.8%, P<0.01). In a separate cervical cancer study, Rose et al. recommended an MVD <26.8 Gy to the lumbosacral and PBM to avoid grade ≥3 leukopenia (20). In female anal cancer patients who are node negative and positive respectively, cutoffs of PBM V15 <68% and V15 <44% were shown to decrease the risk of grade ≥3 leukopenia. Each 1% increase of PBM V15 correlated to a WBC nadir decrease of about 0.02 k/µL (17).
The hematologic benefits of sparing vertebral bone marrow are likewise generalizable. Previously published results on definitive carboplatin and paclitaxel-based chemoradiation therapy (CRT) in patients with non-small cell lung cancer (NSCLC) showed that thoracic vertebral (TV) V30, V20, V5, and MVD were associated with HT (18). Suggested dose for vertebral V20 (56.0% vs. 44.3%) and MVD (23.9 vs. 18.8 Gy) in that cohort of patients to avoid grade ≥3 leukopenia was similar to values found in this study. In the NSCLC study, both a TVV30 of 28% and an MVD of 13.5 Gy were cutoffs associated with a 20% chance of grade ≥3 leukopenia.
Efforts to spare the bone marrow in an attempt to reduce rates of HT can have several implications including improving the tolerance of concurrent chemotherapy, lowering the risk of infections, hospitalizations, fatal complications, and potentially impact survival outcomes in esophageal patients undergoing CRT (21-24). Recent studies have shown that decreases in HT have been observed in esophageal cancer patients receiving proton therapy because of improvement in the distribution of radiation low dose bath (5–15 Gy) compared to IMRT, VMAT, or 3DCRT photon plans (25). Similarly, dosimetric analysis shows that passive scatter proton therapy can decrease HT in stage III NSCLC lung cancer patients, which is statistically correlated with an improvement of TVV10 by around 30% compared with 3DCRT and IMRT treatment (26,27). In turn, improvements in HT may improve adherence to the chemotherapy treatment schedule, which has been shown to significantly correlate with worse patient survival outcomes in NSCLC patients; median OS was 6 months for patients with missed chemotherapy administration compared with 24.3 months for those receiving all doses (P=0.004) (28).
As newer studies seek to improve tumor response and survival by intensifying chemotherapy, establishing reliable bone marrow radiation constraints is necessary to reduce the substantial rates of HT observed (Table 5). A study by Conroy et al. of esophageal cancer patients comparing oxaliplatin, fluorouracil, and leucovorin (FOLFOX) with radiotherapy to the established 5-FU and cisplatin regimen showed that both arms had similar 3-year progression free survival (17–18%) and complete response (55–57%) (13). However, grade ≥3 neutropenia was seen in nearly 30% of patients in both arms and more than 50% of patients experienced leukopenia, anemia, and thrombocytopenia. The phase II CALGB 80803 trial which utilized PET-directed pre-operative CRT initially randomized patients with resectable esophageal cancer either to an induction FOLFOX or carboplatin/paclitaxel arm. PET responders would continue the same chemotherapy concurrently with RT, but PET non-responders would receive concurrent RT with the other chemotherapy arm. Initial results showed a pCR rate 15.6% for those who were initially PET non-responders and 25.2% for PET responders (12). HT rates were similar between patients randomized to either chemotherapy arm. Overall, patients experienced significant HT including 34% grade ≥3 lymphopenia, 13% grade ≥3 neutropenia, 7% grade ≥3 thrombocytopenia, and 6% grade ≥3 anemia.

Full table
Recent modeling studies in solid tumors such as gliomas and pancreas cancers have attributed this significant decrease in WBCs due to irradiation of the circulating blood lymphocytes. However, irradiation of peripheral lymphocyte alone most likely cannot solely contribute to the leukopenia see in esophageal cancer patients receiving chemoradiation. Radiation is known to cause predominantly induce interphase apoptotic death in lymphocytes with intact p53 pathways (29,30). At doses of 2 Gy, in vitro studies determined that the percent of lymphocytes undergoing apoptosis ranges from <10% to 20% (T4 and T8 cells) and around 35% (B cells) when corrected for cells undergoing spontaneous apoptosis without radiation (31,32). Also, while only 35% of the active bone marrow is found in the TV, the dose distribution of esophageal cancer patients treated with IMRT and 3DCRT often does not cover the entirety of T1–T12 vertebra, which suggests that only a small percent of the blood pool is being irradiated (33). Potentially, the constant replenishing of the peripheral blood by active, non-irradiated bone marrow and the fact that lymphocytes only comprises 30% of the body’s total WBC counts dispute the putative role of peripheral lymphocyte irradiation as the main contributor of leukopenia. More likely, a combination of bone marrow toxicity due to CRT and peripheral blood irradiation together contributes to the observed WBC nadirs.
Establishing and adopting bone marrow dose constraints is therefore a meaningful step to reduce HT and may allow for intensification of treatment combinations in esophageal cancer. Incorporation of bone marrow tolerances may lead to decreases in toxicity of therapy by reducing HT complications from patients undergoing chemoradiation and may allow for additional treatment intensification by incorporating additional systemic therapies (34).
Conclusions
The volume of VB receiving low dose irradiation is more important of a predictive factor in limiting HT than the volume receiving high dose irradiation. Adaptation of VB dose constraints may decrease hematologic side effects and improve patient tolerance of therapy and resulting outcomes.
Acknowledgements
Funding: This research was supported by the Biometrics shared resource of Rutgers Cancer Institute of New Jersey (P30CA072720).
Footnote
Conflicts of Interest: Dr. Jabbour receives research funding from Merck and Nestle. The other authors have no conflicts of interest to declare.
Ethical Statement: The protocol for the research project has been approved by a suitably constituted Ethics Committee of the institutional review board within which the work was undertaken and that it conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013. IRB Approved: Robert Wood Johnson Barnabas Health Protocol Number 16-22.
References
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015;1:505-27. [Crossref] [PubMed]
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin 2016;66:271-89. [Crossref] [PubMed]
- Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med 2001;345:725-30. [Crossref] [PubMed]
- Gignoux M, Roussel A, Paillot B, et al. The value of preoperative radiotherapy in esophageal cancer: results of a study of the E.O.R.T.C. World J Surg 1987;11:426-32. [Crossref] [PubMed]
- Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol 2005;6:659-68. [Crossref] [PubMed]
- Stein HJ, Sendler A, Fink U, et al. Multidisciplinary approach to esophageal and gastric cancer. Surg Clin North Am 2000;80:659-82; discussions 683-6.
- Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91:2165-74. [Crossref] [PubMed]
- Schlag PM. Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg 1992;127:1446-50. [Crossref] [PubMed]
- Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979-84. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Lee J, Lin JB, Sun FJ, et al. Dosimetric predictors of acute haematological toxicity in oesophageal cancer patients treated with neoadjuvant chemoradiotherapy. Br J Radiol 2016;89:20160350. [Crossref] [PubMed]
- Goodman KA, Niedzwiecki D, Hall N, et al. Initial results of CALGB 80803 (Alliance): A randomized phase II trial of PET scan-directed combined modality therapy for esophageal cancer. J Clin Oncol 2017;35:1. [Crossref]
- Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol 2014;15:305-14. [Crossref] [PubMed]
- Messager M, Mirabel X, Tresch E, et al. Preoperative chemoradiation with paclitaxel-carboplatin or with fluorouracil-oxaliplatin-folinic acid (FOLFOX) for resectable esophageal and junctional cancer: the PROTECT-1402, randomized phase 2 trial. BMC Cancer 2016;16:318. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Network NCC. Esophageal and Esophagogastric Junction Cancers. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Mell LK, Schomas DA, Salama JK, et al. Association between bone marrow dosimetric parameters and acute hematologic toxicity in anal cancer patients treated with concurrent chemotherapy and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys 2008;70:1431-7. [Crossref] [PubMed]
- Deek MP, Benenati B, Kim S, et al. Thoracic Vertebral Body Irradiation Contributes to Acute Hematologic Toxicity During Chemoradiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2016;94:147-54. [Crossref] [PubMed]
- Mell LK, Kochanski JD, Roeske JC, et al. Dosimetric predictors of acute hematologic toxicity in cervical cancer patients treated with concurrent cisplatin and intensity-modulated pelvic radiotherapy. Int J Radiat Oncol Biol Phys 2006;66:1356-65. [Crossref] [PubMed]
- Rose BS, Liang Y, Lau SK, et al. Correlation between radiation dose to (1)(8)F-FDG-PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int J Radiat Oncol Biol Phys 2012;83:1185-91. [Crossref] [PubMed]
- Mauch P, Constine L, Greenberger J, et al. Hematopoietic stem cell compartment: acute and late effects of radiation therapy and chemotherapy. Int J Radiat Oncol Biol Phys 1995;31:1319-39. [Crossref] [PubMed]
- Rubin P, Landman S, Mayer E, et al. Bone marrow regeneration and extension after extended field irradiation in Hodgkin's disease. Cancer 1973;32:699-711. [Crossref] [PubMed]
- Sykes MP, Chu FC, Savel H, et al. The Effects of Varying Dosages of Irradiation Upon Sternal-Marrow Regeneration. Radiology 1964;83:1084-8. [Crossref] [PubMed]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- Warren S, Hurt CN, Crosby T, et al. Potential of Proton Therapy to Reduce Acute Hematologic Toxicity in Concurrent Chemoradiation Therapy for Esophageal Cancer. Int J Radiat Oncol Biol Phys 2017;99:729-37. [Crossref] [PubMed]
- Sejpal S, Komaki R, Tsao A, et al. Early findings on toxicity of proton beam therapy with concurrent chemotherapy for nonsmall cell lung cancer. Cancer 2011;117:3004-13. [Crossref] [PubMed]
- Nichols RC, Huh SN, Henderson RH, et al. Proton radiation therapy offers reduced normal lung and bone marrow exposure for patients receiving dose-escalated radiation therapy for unresectable stage iii non-small-cell lung cancer: a dosimetric study. Clin Lung Cancer 2011;12:252-7. [Crossref] [PubMed]
- Deek MP, Kim S, Ahmed I, et al. Prognostic Impact of Missed Chemotherapy Doses During Chemoradiation Therapy for Non-Small Cell Lung Cancer. Am J Clin Oncol 2018;41:362-6. [PubMed]
- Payne CM, Bjore CG Jr, Schultz DA. Change in the frequency of apoptosis after low- and high-dose X-irradiation of human lymphocytes. J Leukoc Biol 1992;52:433-40. [Crossref] [PubMed]
- Delic J, Morange M, Magdelenat H. Ubiquitin pathway involvement in human lymphocyte gamma-irradiation-induced apoptosis. Mol Cell Biol 1993;13:4875-83. [Crossref] [PubMed]
- Schmitz A, Bayer J, Dechamps N, et al. Intrinsic susceptibility to radiation-induced apoptosis of human lymphocyte subpopulations. Int J Radiat Oncol Biol Phys 2003;57:769-78. [Crossref] [PubMed]
- Ozsahin M, Ozsahin H, Shi Y, et al. Rapid assay of intrinsic radiosensitivity based on apoptosis in human CD4 and CD8 T-lymphocytes. Int J Radiat Oncol Biol Phys 1997;38:429-40. [Crossref] [PubMed]
- Hayman JA, Callahan JW, Herschtal A, et al. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys 2011;79:847-52. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]


