Evaluation of quality of life and anxiety and depression levels in patients receiving chemotherapy for colorectal cancer: impact of patient education before treatment initiation
Introduction
Although colorectal cancer (CRC) incidence is variable in the different geographical regions of the world, it is the most common cancer of the gastrointestinal system. Globally prostate, lung, and colon cancers are the first three cancers for men; and breast, colon, and lung cancers are the first three cancers seen in women (1,2). According to the Turkish Ministry of Health data for the year 2008, CRC is the third after breast and thyroid cancers in women, and the fourth after lung, prostate and bladder cancers in men (2).
Quality of life is an important outcome measure in the evaluation of health status and treatment efficacy. Health related quality of life is a component of general life quality, which is mainly determined by the individual’s disease, and may also be affected by clinical interventions. Cancer may cause severe and intense problems both because of the disease itself, and/or treatment, which may significantly affect the quality of life of the patients (3).
Emotional stress due to cancer diagnosis may unfavorably affect quality of life. Patients diagnosed with cancer may experience difficulties in dealing and adapting to daily life after the disease. In addition to being a cause of mortality for millions of people, cancer is also a disease with a high risk of psychiatric disorders. The most common psychiatric problems in cancer patients are adaptation disorders and major depression, which may affect patient life quality, self-care, treatment compliance, and severity, progression and response to the treatment in the course of time (4).
Evaluation of quality of life in cancer patients has gained importance because of cancer treatment is toxic and limited treatment efficacy. Quality of life is one of the predictors of survival in CRC patients (5,6). Problems such as diarrhea, constipation, dyspnea, fatigue, insomnia, and economic difficulties may affect physical, emotional, and social well-being of the patients and impairs quality of life (7,8). Treatment modalities such as surgery, chemotherapy, and radiotherapy may adversely affect patient’s life quality (9,10). However chemotherapy favorable affects the quality of life in advanced CRC independently from prolongation of overall life expectancy (11). In the present study, we aimed to evaluate life quality, anxiety and depression levels before and after 6-month follow up period in chemotherapy receiving patients with CRC.
Patients and methods
A total of 65 patients with colon or rectum cancer, who were receiving chemotherapy between years 2010 and 2011, were enrolled in the study. However, 14 patients transferred their treatment schedules to another healthcare service units and 1 patient died, so the study was completed with 50 patients. Patients were followed up for 6 months during their chemotherapy periods. Patients were informed about treatments and their side effects before starting chemotherapy, by one of the researchers, who were working as a training nurse at the chemotherapy unit. A “Questionnaire Form” to collect patient demographic characteristics; the “EORTC QLQ-C30 Scale” and “EQ-5D Scale” to evaluate patients’ life quality; and the “Hospital Anxiety and Depression Measure” to evaluate anxiety and depression state of patients were used as data collecting tools.
Questionnaire form
It includes questions about sociodemographic and disease state of patients such as age, gender, level of education, economic status, disease stage, and treatments they received.
EORTC QLQ-C30 Scale
This scale contains 30 questions and three headings like general well-being, functional difficulties, and symptom control, and it was developed by Aaronson et al. (12). Quality of Life Evaluation Questionnaire containing 30 questions (QLQ-30), which was developed by European Cancer Research and Treatment Center (EORTC), was given to patients to evaluate physical well-being, mental well-being, social life state, metabolic and general states. “Yes” and “No” answers were used only for questions related to physical autonomy in the questionnaire. Answer ‘No’ implies positive, whereas ‘Yes’ implies negative conditions. Answers of other questions are made up of ‘None’, ’Very less’, ‘Fairly’ and ‘Much’. Answers of ‘None’ and ‘Very less’ imply positive, whereas ‘Fair’ and ‘Much’ imply negative conditions. For answers of questions 29 and 30, which inquired about general well-being, “1-3” implied negative; “4” implied moderate; and “5-7” implied positive conditions. Scores related to functional and global health states were calculated according to the manual of scoring for EORTC QLQ-C30. Each parameter had a score between 0 and 100. While high score in the functional scale indicated good health state, high score in the symptom scale indicated excessive symptoms (12,13). The scale was translated into Turkish and its content validation. The reliability study was performed by Beşer and Oz; and the Cronbach alpha coefficient was determined as r=0.9014 (13). These studies validated the scale and showed that it was a reliable tool in assessing quality of life in Turkish patients (12,13).
EQ-5D Scale
EQ-5D is a self-reporting scale. It helps the evaluation of movement, self-care, routine daily activities, feeling of pain/discomfort, and anxiety/depression in five questions. The scale was developed in 1990 by the EuroQoL working group (14). The validation and reliability study of the scale was performed in our country in 2007 by Eser et al. (15). Different health states can be defined as five-digit number sets. For example, 11,111 indicated that there was no problem in five dimensions; 21,213 indicated that there were some problems related to the movement, no problem with self-care, some problems with daily activities, no problem with pain or discomfort, and the presence of severe anxiety or depression. In the present study, the EQ-5D index score was calculated by using value table revealed as the result of the study conducted by York University. There was also a visual analogue scale (VAS) from 0 to 100 points in the scale for participants to self-evaluate their health states (0, the worst health state that could be imagined; 100, the best health state that could be imagined) (14,15).
Hospital anxiety depression (HAD) measure
The HAD was developed to define risk and levels of anxiety and depression, as well as to measure their intensity changes for patients in 1983 by Zigmond and Snaith (16). The validation and reliability study of the scale was performed in Turkey in 1997 by Aydemir O (17). It is used to define the risk group for anxiety and depression in a short time among patients with physical illnesses and who applied to the primary healthcare units rather than diagnosing them. In a total of 14 questions, seven of them (odd numbers) measure anxiety, while the remaining seven (even numbers) measure depression. Answers are scored as in the quartet Likert style between 0 and 3 points. Each item has a different point in the scale. Items no 1, 3, 5, 6, 8, 10, 11 and 13 show a decreasing intensity, and they are scored as 3, 2, 1, and 0. On the other hand, items no 2, 4, 7, 9, 12 and 14 are scored as 0, 1, 2, and 3. For the anxiety sub-scale, points of items no 1, 3, 5, 7, 9, 11 and 13 are added up; points of items no 2, 4, 6, 8, 10, 12 and 14 are added up for the depression sub-scale. The lowest score that patients can receive from both sub-scales is “0 point”, and the highest is “21 points”. The cut-off points for the Turkish form of HAD scale is defined as 10 for the anxiety subscale (HAD-A), and as 7 for the depression sub-scale (HAD-D) (16,17).
Results
The mean age of the patients was 57.04±11.28 years, and 62% of them were male. The median age was 56.50 (min: 29, max: 77) years, and 6% (n=3) of the patients were within 29-36 years; 4% (n=2) were within 37-44 years; 24% (n=12) were within 45-52 years; 26% (n=13) were within 55-60 years; while 40% (n=20) were at and above 60 years. All patients were covered by national social security system, and 72% of them defined their economic level as moderate. Of the patients, 36% was university graduate, while 34% were only primary school graduate. Seventy percent (n=35) of patients had colon cancer, and 30% (n=15) had rectum cancer. Of the patients, 40% (n=20) were in stage II; 32% (n=16) were in stage III; while 28% (n=14) were in stage IV. Chemotherapy regimens used were irinotecan-5-fluorouracil-folinic acid+bevacizumab (folfiri-BEV), oxaliplatin-5-fluorouracil-folinic acid (FOLFOX-4), or 5-fluorouracil-folinic acid (FU/FA). Mean EORTC QLQ-C30 quality of life scores of patients in the first and last courses of the treatment were calculated. Patient scores in all functional fields were high. However, only the emotional functional score was significantly higher in the sixth course of treatment (P<0.05). There was no significant difference between EORTC QLQ-C30 general health conditions and global quality of life scores of patients the first and sixth courses (Tables 1,2).
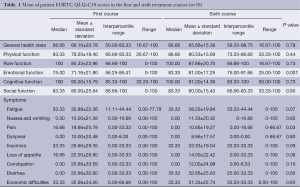
Full table

Full table
Patients reported lower pain scores and experienced less pain in the sixth course of treatment than the first (P<0.05). Other symptom scores of patients were generally higher in the sixth course, but there was no statistically significant difference (P>0.05). The distribution of EQ-5D general health scale responses of patients is given in Table 3. The frequency rates of EQ-5D items were 74% for movement, 86% for self-care, 72% for routine activities, 74% for pain/discomfort, and 74% for anxiety/depression in the sixth course. Patients who had no problem in the five fields during the sixth course had decreased rates of problems than they had in the first course of treatment (82%, 82%, 62%, 58%, and 60% in the same order). The mean EQ-5D health benefit index of patients was between 0.73±0.25 and 0.10-1.0; and it was between 0.83±0.22 and 0.19-1.00 in the sixth course.
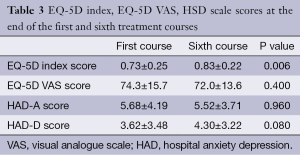
Full table
There was a statistically significant increase in patient quality of life measured in the sixth month by using EQ-5D (P<0.05). The mean EQ-5D VAS score was 74.3±15.7 in the first course, and 72.0±13.6 in the sixth course.
EQ-5D index, EQ-5D VAS and HSD measurement score correlations at the end of first and sixth courses are given in Table 4. According to the table, there was a weak negative correlation in the first course and moderate negative correlation in the sixth course between EQ-5D index and HAD-A scores (P<0.05). Therefore, it may be claimed that as EQ-5D quality of life increases, anxiety and depression of patients decreases. There was a moderate negative correlation in the sixth course of treatment between EQ-5D index and HAD-D scores (P<0.05). Therefore, it may be claimed that as EQ-5D quality of life is increased, depression is decreased. A weak moderate correlation was detected in the quality of life measured by EQ-5D VAS between the first and sixth courses of treatment (P<0.05). A weak negative correlation was reported between general health state/global life quality measured by EORTCQLQ-C30 and HAD-A, HAD-D scores; however, there was a weak correlation between EORTCQLQ-C30 and HAD-A score in the sixth course, and a moderate negative correlation between EORTCQLQ-C30 and HAD-D score in the sixth course (P<0.05). According to data from quality of life scales, it may be claimed that quality of life is better, but anxiety and depression levels are low in the sixth course.
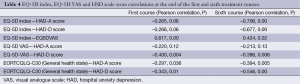
Full table
Discussion
Significant portion of patients who are admitted to hospitals due to CRC are either locally advanced or metastatic at the time of diagnosis, therefore need chemotherapy as apart from their treatment. Chemotherapy reduces risk of recurrence in locally advanced disease and prolongs survival and improves the quality of life in locally advanced and metastatic stage (18-20). In our study patient life quality was better, and the anxiety and depression scores were lower after the sixth course when compared to the first course of treatment. Wan Puteh et al. (21) reported that emotional, cognitive, and social functions of CRC patients with advanced stages were deteriorated; they experienced more pain, dyspnea, diarrhea, and economic difficulties, but as new treatment options were developed, their survival rates increased. Similarly, majority of our patients were also at the advanced stage with deteriorating emotional functional scores at the end of the sixth course of treatment. However, the patients experienced less pain related symptoms at the end of the sixth treatment course. We believe that this is caused by deterioration of emotional states of patients due to the disease and symptoms related to the disease, but intensity and severity of patient symptoms were decreased as a result of the treatment. Yalman et al. (20) reported that patients with rectal cancer that adjuvant chemotherapy after surgery increased survival rate by 5 years. O’Connell et al. (19) reported that 6-month adjuvant chemotherapy given post-operatively had positive effects on patient survival rates, and on prevention of tumor recurrence. In addition to the six course of chemotherapy in our study, patient training and regular follow up had positive impacts on the patient’s dealing with the disease and on the treatment period, and also positively affected patient life quality. In previous studies, it was also reported that psychosocial support, training and regular follow up of cancer patients receiving chemotherapy improved emotional, physical well-being, and quality of life of the patients (7,22). In our study, a moderate negative correlation was determined in the sixth course of treatment between EQ-5D life quality and HAD-A, and HASD-D scale scores (P<0.05). A weak negative correlation was reported between general health state/global life quality measured by EORTCQLQ-C30 and HAD-A, HAD-D scores; however, there was a weak correlation between EORTCQLQ-C30 and HAD-A score in the sixth course, and a moderate negative correlation between EORTCQLQ-C30 and HAD-D score in the sixth course (P<0.05). In the study conducted by Kutlu et al. (4), data obtained in life quality fields such as general health and life satisfaction, physical health, social relationships, psychological health and social environment were significantly higher in patients without depression than in those with depression, when life quality scores and depression state were compared. Beser and Oz (13) reported in their study that general well-being and physical function score out of the life quality subgroups before and after chemotherapy were lower in patients with depression than their counterparts (P<0.05). Alacacioglu et al. (23) reported that EORTC-QLQ-C30 global life quality scores were significantly lower in CRC patients with high anxiety and depression levels. Similar to our study, Tsunoda et al. (24) reported that depression affected EORTC-QLQ-C30 global life quality more strongly than anxiety in CRC patients.
Conclusions
In conclusion, our study results indicate that patient life quality is better at the end of the sixth treatment course than the first. We believe that regular chemotherapy and patient follow up, and patient education before treatment initiation are important in the favorable outcomes.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [PubMed]
- Department of Cancer, Public Health Agency of Turkey. Cancer incidences in Turkey (2009). Available online: http://www.kanser.gov.tr/. Last date of access 7.10, 2013.
- Altıparmak S, Fadıloğlu Ç, Gürsoy ŞT, et al. Kemoterapi tedavisi alan akciğer kanserli hastalarda öz bakım gücü ve yaşam kalitesi ilişkisi. Ege Tıp Dergisi 2011;50:95-102.
- Kutlu R, Çivi S, Börüban ME, et al. Kanserli hastalarda depresyon ve yaşam kalitesini etkileyen faktörler. Selçuk Üniv Tıp Derg 2011;27:149-53.
- Arslan S, Bölükbaş N. Kanserli hastalarda yaşam kalitesinin değerlendirilmesi. Atatürk Üniversitesi Hemşirelik Yüksekokulu Dergisi 2003;6:38-47.
- Yost KJ, Hahn EA, Zaslavsky AM, et al. Predictors of health-related quality of life in patients with colorectal cancer. Health Qual Life Outcomes 2008;6:66. [PubMed]
- Hoon LS, Chi Sally CW, Hong-Gu H. Effect of psychosocial intervene tions on outcomes of patients with colorectal cancer: A review of the literature. Eur J Oncol Nurs 2013;17:883-91. [PubMed]
- Lerdkiattikorn P, Tantai N, Chaikledkaew U, et al. Quality of life among stage III colon cancer patients receiving oral and intravenous chemotherapy regimens in Thailand. Mahidol University Journal of Pharmaceutical Sciences 2012;39:41-3.
- Graça Pereira M, Figueiredo AP, Fincham FD. Anxiety, depression, traumatic stress and quality of life in colorectal cancer after different treatments: A study with Portuguese patients and their partners. Eur J Oncol Nurs 2012;16:227-32. [PubMed]
- Kucukoner M, Kaplan MA, Inal A, et al. Colorectal cancers: 12 year-results of a single center. JCEI 2013;4:208-12.
- Maisey NR, Norman A, Watson M, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer 2002;38:1351-7. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [PubMed]
- Beser N, Öz F. Kemoterapi alan lenfomalı hastaların anksiyete-depresyon düzeyleri ve yaşam kalitesi. C Ü Hemşirelik Yüksekokulu Dergisi 2003;7:47-58.
- EuroQol Group Executive Office, On behalf of the EuroQol Group. EQ-5D-5L User Guide, Basic information on how to use the EQ-5D-5L instrument 2011. Available online: ;
- Eser E, Dinc G, Cambaz S, et al. Population standards and psychometric characteritics of EURO-QoL (EQ-5D) index: Manisa city population sample. 2nd Quality of Life in Health Congress Abstract Book, Izmir-Turkey, 2007:78.
- Zigmond AS, Snaith PR. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. [PubMed]
- Aydemir Ö. Validity and reliability of Turkish version of hospital anxiety and depression scale. Turk Psikiyatri Derg 1997;8:280-7.
- Er Ö, Coşkun HŞ, Altınbaş M, et al. İleri evre kolorektal kan- serlerin tedavisinde Erciyes Üniversitesi sonuçları. Erciyes Tıp Dergisi 2003;25:144-9.
- O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246-50. [PubMed]
- Yalman D, Demirci S, Bolukbasi Y, et al. Postoperative radiotherapy in rectal cancer: Long term results of patients. Hepatogastroenterology 2010;57:1099-105. [PubMed]
- Wan Puteh SE, Saad NM, Aljunid SM, et al. Quality of life in Malaysian colorectal cancer patients. Asia Pac Psychiatry 2013;5 Suppl 1:110-7. [PubMed]
- Aslan Ö, Vural H, KömürcüŞ, et al. Kemoterapi alan kanser hastalarına verilen eğitimin kemoterapi semptomlarına etkisi. C Ü Hemşirelik Yüksekokulu Dergisi, 2006;10:15-28.
- Alacacioglu A, Binicier O, Gungor O, et al. Quality of life, anxiety, and depression in Turkish colorectal cancer patients. Support Care Cancer 2010;18:417-21. [PubMed]
- Tsunoda A, Nakao K, Hiratsuka K, et al. Anxiety, depression and quality of life in colorectal cancer patients. Int J Clin Oncol 2005;10:411-7. [PubMed]


