Quality of life and hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. It is the 5th most common cancer worldwide and the 3rd leading cause of cancer-related deaths (1,2). This tumor is relatively uncommon in the United States although its incidence has been increasing due to the increased burden of hepatitis C infection. Risk factors associated with HCC include cirrhosis, hepatitis B and C infections and alcohol intake. Cirrhosis is present in 80-90% of HCC patients and is thereby the single largest risk factor (3). Non-alcoholic steatohepatitis has also emerged as an important cause of HCC (4).
The optimal therapeutic option for HCC is liver transplantation as it treats both the neoplasm and any underlying cirrhosis; however, only 20% of patients diagnosed with HCC are candidates for transplantation (5). Other treatment options include surgical resection for patients with resectable HCC for those with preserved liver function, locoablative treatments for small, solitary HCC, liver-directed therapies for multifocal HCC without contraindications and systemic therapy for metastatic or multifocal HCC that is associated with limited hepatic reserve or portal vein involvement (2). Quality of life (QoL) after major surgical resection in patients with cancer is well known, and especially important, given the morbidities of liver resection and since recurrence is the natural course of the disease for many due to their underlying liver disease. Chemoembolization can also cause considerable pain/discomfort immediately after the procedure and also cause decompensation of liver function and impact patient’s QoL. Unfortunately 80% of the patients are unable to undergo surgical resection or transplantation (6). And non-surgical treatments, like transcatheter chemo-embolization or chemotherapy have a limited impact on patient survival that remains between 6 months to a year in majority of cases (2,7-10). The only approved therapy currently for HCC is sorafenib, an oral targeted agent with considerable off target side effects in ~80% of patients. Despite advances in treatment over the past decade, overall prognosis remains poor. Population-based studies in the United States indicate that 1- and 3-year survival rates for patients with HCC are approximately 20% and 5%, respectively, with a median survival of 8 months. There is therefore an urgent need for novel therapies that are being developed to palliate symptoms and prolong life.
QoL is considered important for patient outcome and is considered as important as disease-free survival and overall survival and should be an endpoint like response rate and time to progression (11-13). Health-related quality of life (HRQoL) subjectively perceived by the patient, is becoming a major outcome in the evaluation of any therapeutic intervention, mainly in patients with chronic or poorly curable diseases, where the aim of the interventions is to maintain patients either symptom-free and community-living for a long time, or to reduce the distress of the disease. Patients with HCC report several symptoms which are severe enough to affect the QoL like, sleep disorders, sexual dysfunction, ascites, gynecomastia, pruritis, fatigue, muscle cramps. The HRQoL indicators that have been used in trials thus far are based on these symptoms (14).
Goal of therapy in patients who present with symptoms is palliation but limited data exist on whether this goal is achieved with chosen therapies. Having this information could influence provider and patient decision-making given the short survival and many side-effects of therapy. Hence, HRQoL is of paramount importance in patients diagnosed with HCC enrolled on trials as this sparse trial eligible patient population data forms the basis for therapeutic decision-making for many others who are more symptomatic and hence in greater need for interventions that improve their QoL. HRQoL results may be more relevant than length of life, as patients are often more concerned about life-quality than longevity (15). HRQoL is an important aspect of palliative care and has been acknowledged as an important end point in several randomized clinical trials and clinical practice (16,17).
In HCC, both cancer and its treatment are severely debilitating and the need to consider their impact upon HRQoL when making patient management or treatment decision is well-accepted (18). Hence, we conducted a review of literature on all the studies published in the last 13 years assessing QoL in patients with HCC as a primary or secondary end point to help guide clinicians across many disciplines who are designing trials for these patients.
Patients and methods
We searched PUBMED for all English-language publications that dealt with HRQoL in HCC using the following terms: health utility, health status, health status indicators, activities of daily living (ADLs), QoL and HCC. Studies were included if they had been published in the last 13 years, and if patients were treated with surgery, hepatic arterial infusion, chemotherapy, radionuclide therapy, or observation. All trials included some QoL or functional measure as an outcome: either primary or secondary or as an independent variable.
The primary aim of this study was to describe the tools being used to assess HRQoL in patients with HCC and to summarize how to use and interpret data gathered using these tools.
Results
In our search, we found 25 relevant articles published in the last 13 years [2001-2013] that identified HRQoL as a primary end point (Table 1). We also found 20 other articles that had HRQoL as one of their secondary end points (Table 2). There were an additional four meta-analysis that met inclusion criteria for our search. In 12 of these studies, the numbers of patients with HCC were less than 50. In the cross-sectional studies, we can compare QoL in HCC patients who received different treatment modalities, such as, surgery, transarterial embolization, local liver-directed treatment, chemotherapy, or just supportive care. In the longitudinal study, we can compare the QoL in patients before and after the treatment. In our tables, the various HRQoL indicators included assessed general symptoms of well-being or liver-specific symptoms like fatigue, diarrhea, back pain, jaundice, and impairment in sexual functions. These QoL indicators are significantly impaired in HCC patients, and this can help in better addressing the management of these patients with HCC by the physicians in a patient-centered model of care.
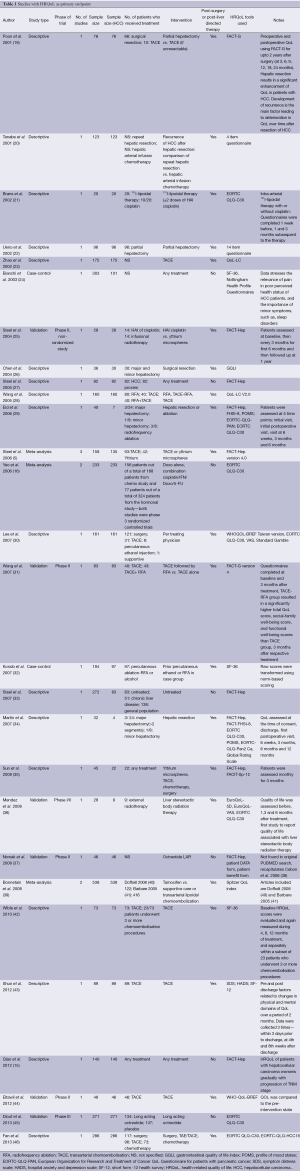
Full table
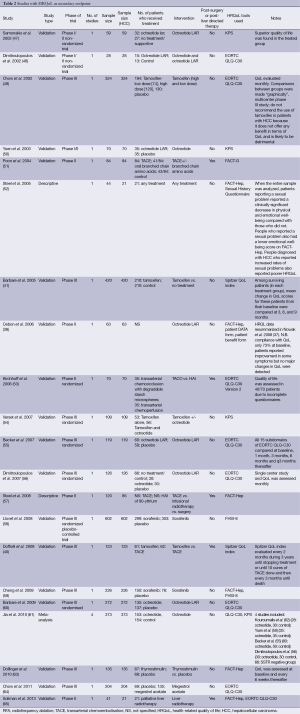
Full table
Commonly used tools
The commonly used HRQoL tools included European Organization for Research and Treatment Quality of Cancer Questionnaire - Core 30 (EORTC QLQ-C30), Functional Assessment of Cancer Therapy-Hepatobiliary questionnaire (FACT-Hep), Functional Assessment of Cancer Therapy Hepatobiliary Symptom Index (FHSI-8), Functional Assessment of Cancer Therapy-General (FACT-G), Spitzer QoL index, World Health Organization Quality of Life- BREF (WHO-BREF), Short Form 36 (SF-36) and European Organization for Research and treatment of Cancer Qualtiy of Life Questionnaire - Pancreatic Cancer (EORTC QLQ-PAN).
EORTC QLQ-C30 was the most widely utilized tool, with 15 publications and 4 phase I/II and 6 phase III clinical trials identified.
FACT-Hep has also been widely published, with 14 publications identified in our literature review. The use in clinical trials, however, is not as extensive, with only five phase I/II trials and two phase III trials identified.
FHSI-8 was used in four publications with two phase III clinical trials.
FACT-G was used in three publications (two phase I/II clinical trials), SF-36 was used in three publications, WHO-BREF was used in two publications, Spitzer QoL index was used in two publications (two phase III trials), EORTC QLQ-PAN was used in two publications (Table 3).
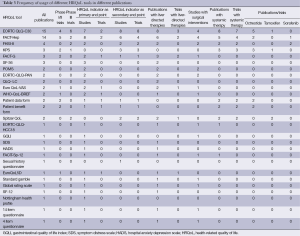
Full table
HRQoL as primary or secondary endpoint
In our analysis, there were 25 publications (six trials) with HRQoL as a primary endpoint. Most commonly used tool as a primary endpoint was FACT-Hep (eight publications with two trials) followed by EORTC QLQ-C30 (seven publications with two trials). A total of 20 publications (18 trials) used HRQoL as a secondary endpoint. Most commonly used tool as a secondary endpoint was EORTC QLQ-C30 (eight publications, all of which were trials) followed by FACT-Hep (six publications including four trials). Most of the trials (18 out of 24) assessed HRQoL as a secondary endpoint with EORTC QLQ-C30 being the most commonly used tool (ten trials) followed by FACT-Hep (six trials). Several publications using these tools were data from case series rather than prospective trials.
Tools used to measure clinical outcome post-surgical intervention
In our analysis, there were ten publications with surgical intervention (hepatic resection). The most widely used tools were FACT-Hep (four publications) and EORTC QLQ-C30 (four publications). However, none of the trials during this period assessed HRQoL as an outcome to measure the impact of surgical intervention.
Tools used to measure clinical outcome post liver-directed therapies
There were 23 publications (seven trials) where liver-directed therapies were used [liver-directed therapies include: transarterial chemoembolization (TACE)/infusional radiotherapy/hepatic resection/percutaneous ethanol ablation/radiofrequency ablation (RFA)/liver stereotactic body radiation]. The most widely used tools were EORTC QLQ-C30 (eight publications including three phase I/II trials), FACT-Hep (six publications including two phase II trials).
Hence, for surgical interventions and locoregional therapies, the tools most frequently used were EORTC QLQ-C30 and FACT-Hep. The additional questions they addressed were liver-specific questions like ascites, weight loss, loss of bowel control, back pain, fatigue, jaundice and pruritis.
Tools used to measure clinical outcome post-systemic/medical intervention
There were about 19 publications (17 trials) where HRQoL was used to monitor the impact of systemic intervention (octreotide, tamoxifen, sorafenib, thymostimulin, megestrol, chemotherapy). Majority of the trials assessed octreotide as a medical intervention (ten publications, that is, five phase I/II and five phase III trials) followed by tamoxifen (four publications, that is, one phase I/II and three phase III trials) and sorafenib (two publications, that is, two phase III trials). The most commonly used tool was EORTC QLQ-C30 (eight publications including seven trials) followed by FACT-Hep (five publications including four trials) and KPS (three publications, all of which were trials).
Studies which used generic HRQoL indices
Generic HRQoL indices (EORTC QLQ-C30, KPS, FACT-G, SF-36, Profile Of Mood States, EuroQoL-Visual Analogue Scale, WHO-QoL BREF, Patient DATA form, Patient BENEFIT form, Spitzer QoL index, Symptom Distress Scale, Hospital Anxiety Depression Scale, FACIT-Sp-12, sexual history questionnaire, Euro-QoL-5D, Standard gamble, SF-12, Nottingham Health Profile, 14 and 4 item questionnaire) were used in 33 publications, which included 12 phase I/II trials and 8 phase III trials.
Studies which used liver-specific indices
Liver-specific HRQoL indices which included specific questions like ascites, weight loss, diarrhea, constipation, jaundice, pruritis (that is, FACT-Hep, FHSI-8, EORTC QLQ-PAN, QLQ-LC, EORTC QLQ-HCC18, Gastrointestinal Quality of Life Index, Global Rating Scale) were used in 19 publications which included 4 phase I/II trials and 3 phase III trials.
Discussion
There is a wide variety of symptom presentation in advanced HCC; compensated patients may be asymptomatic for months or decades. In patients who are symptomatic from HCC, the most common presenting clinical features are right upper quadrant pain, weight loss, anemia or erythrocytosis. These are often superimposed on signs of cirrhosis (e.g., jaundice, palmar erythema, gynecomastia) and portal hypertension (e.g., ascites, varices), and may also be associated with increase in liver transaminases (2). It has a significant impact on the patient’s functioning and well-being. Emotional concerns associated with the disease and treatment give rise to anxiety in patients. The QoL, including physical, emotional, and functional well-being are significantly affected because of the complications and extra-hepatic manifestations of advanced disease (66). Trial eligible HCC patients are few, as most patients have liver dysfunction to some degree and aren’t candidates for many available treatments. In addition, toxicities of therapies employed are significant.
QoL is defined as people’s perceptions of their position in life in the context of the culture and value systems in which they live, and in relation to their goals, expectations, standards, and concerns (67). Patients often ask providers what to expect in terms of their QoL when choosing a therapy, especially when the survival is short even with treatment. Unfortunately, very few trials have used validated HRQoL tools, hence, patterns of clinical decision-making are more often guided by available data on toxicity of treatment which is not a true surrogate of QoL because it does not assess the impact of treatment on existing symptoms or the patient’s perception of their health/ well-being. HRQoL questionnaires potentially play a significant role in bringing the patient’s voice to evidence-based health care. However, to fully realize this potential, HRQoL outcomes need to be interpreted to make decisions about treatment. Such decisions are made at both the individual level, when a patient (along with the patient’s clinician and care team) chooses among treatment options, and the group level, when clinical research is conducted to test the effectiveness of new treatments relative to current routine treatment (68,69). New treatments that improve the HRQoL relative to the current best treatment may be able to change policies and practices regarding treatment of those conditions.
HRQoL is multifaceted and subjective, and there are a large number and wide range of measurement scales, each of which has a different scale. The two most commonly used cancer-specific instruments are the EORTC QLQ-C30 (70) and the FACT-G (71).
The development of valid and reliable HRQoL instruments is an essential part of quantifying the physical, social and psychological distress associated with cancer diagnosis and its treatment. Useful tools must satisfy the basic psychometric principles of validity and reliability in the patient population being studied (Table 4). Additional desirable features of HRQoL instruments include patient self-administration, multiple dimensions, low respondent burden, and the ability to obtain subscale scores and an overall score (38). With the recent expansion of interest in measuring QoL, there has been a proliferation of validated tools for the measurement of various aspects of HRQoL. A recent review of an online repository of HRQoL tools (proquolid.org) identified 70 neoplasia-specific questionnaires. Selection of an appropriate tool requires considering the specific population being studied, prior precedence for a given tool in the given population, and means by which both clinical significance and statistical significance can be inferred for the given tool.

Full table
Details of the development, measures, interpretation of the various tools is in appendix A. Here we briefly discuss the two most commonly used tools and the tool used in the landmark SHARP trial as a primary endpoint in advanced HCC.
The EORTC QLQ-C30 was originally devised by Aaronson et al. in the Netherlands (70) and the FACT G was developed by Cella et al. in the United States (71). Both of these instruments have undergone vigorous validation and have been translated and tested in more than 40 different languages. They are therefore suitable to be used in cancer clinical trials and allow for cross-cultural comparisons. Functional Hepatobiliary symptom index (FHSI-8) is an eight item questionnaire to assess symptoms that measure lack of energy, fatigue, stomach pain/discomfort, pain, back pain, weight loss, nausea, jaundice also developed by Dr. Cella’s group in addition to the FACT tools.
The EORTC QLQ C30 questionnaire is a cancer-specific self-administered structured questionnaire designed for use in clinical trials. It is an integrated system that assesses the HRQoL of cancer patients. It includes five functional scales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, nausea or vomiting, and pain), global health status, and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). High scores on the functional scales indicate a high level of functioning and high scores on the global health status indicate a high QoL; however, high scores on the symptom scales/items indicate high levels of health problems. Brans et al. evaluated the feasibility of using this questionnaire following radionuclide liver-directed therapy using palliative 131I-lipiodol therapy for HCC. In 20 patients treated with locoregional, intra-arterial 131I-lipiodol therapy with or without cisplatin, they found (I) a number of important scales, i.e., overall QoL, physical functioning and pain, worsened between 0 and 3 months after 131I-lipiodol therapy, irrespective of tumor response; and (II) the occurrence of clinical side-effects was associated with a negative impact on QoL and physical functioning 1 and 3 months after 131I-lipiodol, demonstrating that the value of this tool is assessing clinical impact following what is considered by most to be a well-tolerated/non-toxic treatment.
The Functional Assessment of Cancer Therapy-Hepatobiliary (FACT-Hep) is a cancer-specific version of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system (72). The FACT-Hep contains the original FACT-General (FACT-G) scales that include a 27-item compilation of general questions divided into four primary QoL domains: physical, social/family, emotional, and functional well-being. An additional 18 questions that assess symptom and QoL concerns pertinent to patients with hepatobiliary cancer were included. In a clinical trial assessing benefit from octreotide in HCC given that 56% of patients have receptor expression detectable by scintigraphy, Cebon et al. used this tool to assess impact on QoL. One patient of 63 had a partial response and overall survival was 8 months, few grade ¾ side effects were reported, but no major changes in QoL were detected using the FACT-Hep tool that allowed better interpretation of the results.
In the SHARP trial by Llovet et al. 2008, a new drug sorafenib was assessed in advanced HCC (58). In this study, 602 patients were randomly assigned to either drug or placebo group. The HRQoL indicator FHSI-8 questionnaire was used to assess the primary outcome, that is, median time to symptomatic progression, which was defined as either a decrease of four or more points from the baseline score on the FHSI-8 questionnaire or an ECOG status of four or death. No significant differences were observed between the sorafenib and the placebo groups. Symptoms related to the toxic effects of the drug or effect of response to tumor-related symptoms might have influenced the outcomes of the FHSI-8 questionnaire. The lack of a significant difference in responses to the FHSI-8 questionnaire might reflect the effect of the reporting of sorafenib’s toxic effects by the patients, insensitive measurement tool, lack of power for the TTSP endpoint, or absence of any benefit from sorafenib or lack of the study design to be powered for this endpoint (58).
Thus, in each of the three trials discussed above, although the sample sizes are different (20, 63 and 602 respectively), interventions tested were different and the tools used were different, clinically meaningful data was added that would guide treatment decision-making. Radiolabeled liver-directed therapies, even when successful can have a significant negative impact due to side effects, a relatively benign therapy such as octreotide may not positively impact QoL even though side effects are few, and an oral drug like sorafenib that adds meaningful survival benefit may not improve existing symptoms and patient’s perception of well-being.
Conclusions
Historically, outcome measurements in oncology have been limited to survival and treatment toxicity. However, nowadays it has been widely accepted by clinicians that QoL is an important prognostic indicator, as important as length of survival. The available literature on HRQoL is limited in hepatobiliary cancers and no gold standard exists for measuring HRQoL. However, in the last two decades there has been development of several HRQoL instruments. The QoL components measured in the various HRQoL questionnaires and their analysis is presented in the discussion to help clinicians understand and interpret the results from published studies. Here we summarize the take home points- how available data can help guide future trial design and highlight areas of need where additional validation or QoL data are badly needed.
In our literature review, the HRQoL indicators that have been most frequently used are EORTC QLQ-C30 which has been used in 15 publications with 4 phase I/II trials and 6 phase III trials, followed by FACT-Hep which has been used in 14 publications which include 5 phase I/II trials and 2 phase III trials. More studies need to incorporate these tools as they have been extensively used making it easier to compare QoL outcomes between similar interventions, and the broad range of QoL elements studied make them suitable for studies where therapies are toxic and survival is poor.
In addition to being validated as a primary outcome measure, the same tools (EORTC QLQ-C30 and FACT-Hep) have also been most commonly used as secondary endpoint assessment tools to measure the impact of different interventions.
Liver specific QoL is an important variable especially when studying liver-directed therapy outcomes. Most of the studies that met our inclusion criteria, used generic HRQoL indices (33/45 studies-both trial and case series), however, liver-specific indices were not used that frequently (19/45 studies). More studies in the future need to incorporate liver-specific indices as endpoints in HCC patients.
Analyzing the trend over the past 13 years, there has been no bias towards a particular HRQoL tool to assess the impact of a particular therapeutic intervention. Although surgical or liver-directed interventions are most likely to have a negative short-term impact on QoL with a higher potential for long-term favorable outcomes, and systemic therapies are offered to individuals who are more symptomatic, have a shorter survival, the tools used have been the same. Although the populations getting potentially curative vs. palliative therapies vary in their expectations and their outlook towards their cancer, having the same tool provides a uniformity of QoL assessment. HRQoL indicators were chosen as an endpoint for over 50% of studies evaluating liver-directed therapies (23 out of 45 publications). This may be reflection of the time period included in the study inclusion 2001-2013. As more than 80% of patients have multifocal/advanced disease and no therapy had been shown to improve survival in this setting until 2007, hence rationalizing selection of liver-directed therapies that favorably impacted QoL was a focus. Sorafenib is the only systemic therapy that showed improved survival and received FDA approval for treatment of advanced hepatocellular cancer in Nov 2007, although the trial did not meet its primary QoL endpoint. Survival even with sorafenib remains under one year and has led to a surge of new systemic therapy trials that began following the approval of sorafenib. As these studies get completed and published, future reviews of QoL endpoint trials maybe biased towards systemic therapy or combination therapy trials.
Very few publications (10/45, that is, 22%) addressed HRQoL indicators as endpoints for post-surgical interventions maybe a reflection of the lower frequency of patients being surgical candidates and highlight the need for greater awareness of the value of these tools in the surgical community and closer collaborations between surgical and other supportive care providers with greater familiarity with such endpoints. Of the 45 publications utilizing HRQoL endpoints, only 24 were clinical trials. More trials (17/24, 71%) using systemic therapies (medications) incorporated HRQoL as endpoints, compared to trials of liver-directed therapies (7/24, 29%). This may represent a publication bias, i.e., novel therapy evaluations are more readily publishable, while trials of routinely used interventions are published only when compared to another intervention (chemoembolization versus radioembolization) and are able to be conducted only in high volume centers as there is a lot of variability in technique and patient selection between centers. Unfortunately, funding for QoL studies as a primary endpoint is sparse as well.
We have provided a summary of HRQoL instruments that are available and being used in patients with HCC to guide future HCC trial design and interpretation of existing QoL data.
Appendix A: quality of life tools used in HCC trials/publications
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30)
EORTC QLQ-C30 is the instrument most frequently used to measure the quality of life of cancer patients (70). In 1986, the EORTC study group on health-related quality of life (HRQoL) started a research program addressed to the development of instruments for the assessment of HRQoL in international oncology trials. This working group initially developed the EORTC QLQ-C36 which has been further refined and designated EORTC QLQ-C30. The EORTC QLQ-C30 questionnaire is a cancer-specific self-administered structured questionnaire designed for use in clinical trials. It is an integrated system that assesses the HRQoL of cancer patients. It includes five functional scales (physical, role, emotional, cognitive, and social), three symptom scales (fatigue, nausea or vomiting, and pain), global health status, and six single items (dyspnea, insomnia, appetite loss, constipation, diarrhea, and financial difficulties). High scores on the functional scales indicate a high level of functioning and high scores on the global health status indicate a high quality of life; however, high scores on the symptom scales/items indicate high levels of health problems (73). The EORTC QLQ-C30 has been translated into more than 60 languages. It is said to be applicable across a range of cultural settings (74). There is sufficient evidence to support its reliability and validity (70,73).
The development of the provisional module of EORTC QLQ-C30 that assesses quality of life in patients with HCC, was performed according to the EORTC quality of life Group (QLG) published guidelines for questionnaire development.
The EORTC QLQ-HCC18 has been developed using standard guidelines. It is designed for use with the QLQ-C30 core instrument to assess all major dimensions of HRQoL in patients with HCC. The content of the questionnaire has not only been derived from published literature but also from health professionals dealing with these patients and from the patients themselves. The QLQ-HCC18 contains 18 items hypothesized to include five multi-item symptom scales: fatigue, jaundice, nutrition, pain, and fever, two single-item symptom scales—abdominal swelling and sexual interest—and one multi-item functional scale—body image (74).
Although it has primarily been developed within two language groups, it is the first questionnaire to include patients from the East and West and has the potential for use in international trials in HCC. The development of EORTC QLQ-HCC18 has primarily involved patients from the UK, Taiwan and China and is currently available in Arabic, Chinese, English and Taiwanese (74). This instrument is currently being validated and has not been used in any clinical trials. As per Chie et al., EORTC QLQ-HCC18 can be used as a supplementary module for EORTC QLQ-C30 in clinical trials for patients with HCC.
The Functional Assessment of Cancer Therapy–General (FACT-G)
FACT-G is a widely used quality of life instrument for cancer patients. The questionnaire was originally developed using semi-structured interviews of patients and oncology professionals to generate instrument items (71). It is a 27 item self-report instrument (version IV) that assesses four dimensions of HRQoL: physical, social/family, emotional and functional well-being. The 27 general questions are applicable to all patients with all types of cancer and have been used in chronic conditions. It takes about ten minutes to complete. Additional disease, treatment and condition-specific subscales have also been developed to assess symptoms specific to certain diseases. The FACT-G has demonstrated both discriminant and convergent validity with test-retest correlations ranging from 0.82 to 0.92 (75).
Functional Assessment of Cancer Therapy- Hepatobiliary (FACT-Hep)
From June 1997 to April 1998, FACT-Hep scale was developed and validated. The FACT-Hep is a cancer-specific version of the Functional Assessment of Chronic Illness Therapy (FACIT) measurement system (72). The FACT-Hep is developed specifically for use in patients with hepatobiliary cancers. It contains the original FACT-General (FACT-G) scales that include a 27-item compilation of general questions divided into four primary QoL domains: physical, social/family, emotional, and functional well-being. An additional 18 questions that assess symptom and QoL concerns pertinent to patients with hepatobiliary cancer were included. All the disease-specific QoL tools in the FACT system include the original FACT-G as well as a disease-specific subscale. All items are scored from 0-4, with higher overall and subscale scores indicating better QoL. It has excellent test-retest reliability (Cronbach’s alpha range, 0.81 to 0.94) (75).
Functional Hepatobiliary Symptom Index-8 (FHSI-8) Questionnaire
FHSI-8 is an 8-item index which was constructed based on the clinical importance ratings of an international sample of hepatobiliary cancer specialists. Validation of the eight items demonstrated that these items have adequate reliability and validity to assess the important symptoms in this population. FHSI-8 demonstrated good internal consistency, test-retest reliability and convergent validity. The advantage of FHSI-8 is its shorter length. The investigator can decide whether to select the briefer assessment with some loss to precision—FHSI-8 or to select the longer version for more accurate assessment—FACT-Hep. Sample size might play a role in the selection. As the sample size increases, more preference may be given to FHSI-8 questionnaire for assessment. However, for more accurate individual assessment, one may be inclined towards using FACT-Hep (45 items) due to its more favorable internal consistency (76).
Patient DATA form is designed to measure aspects of HRQoL that are relevant to people with advanced cancer. It assesses 24 aspects of health related quality of life using simple worded items listed on a single page: 16 physical and emotional symptoms of cancer rated on numeric scale from zero (no trouble at all) to ten (worst I can imagine) and eight aspects of well-being rated from zero (worst possible) to ten (best possible). The patient DATA form is designed to be rapidly and easily interpreted. Items are arranged in two blocks: symptoms and dysfunctions where high scores reflect worse QoL or more severe symptoms. However, in aspects of well-being, higher scores reflect better well-being and quality of life.
The patient benefit form is a health transition scale that rates changes in the same aspects assessed with the patient data form using a similar, one page format. The FACT-Hep baseline scores are calculated for each patient according to the FACT scoring guidelines. Domain and total scores are linearly transformed to a score from 0 (worst HRQL) to 100 (best HRQL) to make interpretations and comparisons easy (38).
Short Form-36 (SF-36) survey
SF-36 Survey is one of the most widely used HRQoL instruments in the US. It is a generic questionnaire that measures two major health concepts (physical and mental health) with 36 questions and eight multi-item scales: physical functioning, social functioning, vitality, role limitations due to emotional problems, bodily pain, role limitations due to physical problems, mental health, and general health (77). Each domain is scored on a range from 0 to an optimal result of 100. An additional one item measurement of self-evaluated change in health status is also available (78).
Extensive psychometric testing has been conducted on the SF-36 in the United States. With very few exceptions, published reliability statistics have exceeded the minimum standard of 0.70 recommended for measures used in group comparisons in more than 25 studies. Most of the reliability statistics have exceeded 0.80 and reliability statistics for physical and mental summary scores usually have exceeded 0.90. The validity of the SF-36 has been compared with that of other widely used generic health surveys. Comparisons have shown that the SF-36 includes eight of the most frequently measured health concepts. However the validity and the interpretation of each of the eight scales and the two summary measures have been shown to differ. The SF-36 is suitable for self administration, computerized administration or administration by a trained interviewer in person or by telephone, to persons aged 14 years and older. It can be administered in 5-10 minutes with a high degree of acceptability and data quality.
WHOQOL-100 and WHOQOL-BREF
The WHO group developed two instruments for measuring quality of life WHOQOL-100 and the WHOQOL-BREF. The instruments can be administered in a variety of settings and can be used to compare QoL in different populations and countries. The WHOQOL-100 consists of 24 subscales to a total of 100 items. The subscales include six domains: physical (energy and fatigue, sleep and rest and pain and discomfort), psychological (body image and appearance, negative feelings, positive feelings, self-esteem), independence (in activities of daily living), social (personal relationship, social support), environment (physical safety and security, financial resources) and spiritual.
It is one of the best-known instruments that has been developed for cross-cultural comparisons of quality of life and is available in more than 40 languages (79).
It is a shortened version of the WHOQOL-100 that looks at four quality of life profiles, using all available data from the field trial version of the WHOQOL-100 (79).
It is a 26-item instrument consisting of four domains: physical health (seven items), psychological health (six items), social relationships (three items) and environmental health (eight items); and two overall QoL and general health items that are used to measure an individual’s overall satisfaction with life and general sense of personal well-being. The physical health domain includes items on mobility, daily activities, functional capacity and energy, pain, and sleep. The psychological domain measures self-image, negative thoughts, positive attitudes, self-esteem, mentality, learning ability, memory and concentration, religion, and the mental status. The social relationships domain contains questions on personal relationships, social support, and sex life. The environmental health domain covers issues related to financial resources, safety, health and social services, living, physical environment, opportunities to acquire new skills and knowledge, recreation, general environment (noise, air pollution, etc.), and transportation (80).
The WHOQOL-BREF domain scores have good validity (discriminant and content) and good reliability (internal consistency and test retest). Cronbach’s alpha coefficients were acceptable (0.7) for all subscales (81).
Spitzer QoL Index (SQLI)
SQLI covers five dimensions of quality of life (activity, daily living, health, support of family and friends and outlook). It has been designed to be used by physicians to help them assess the risks and benefits of various treatments. This QoL index was validated in 1981 on the basis of pretests and validation tests by more than 150 physicians to rate 879 patients (82). The time of completion is about one minute. The SQLI has convergent discriminant and content validity among cancer patients. Assessment of internal consistency has demonstrated a high coefficient (Cronbach’s α =0.775) and the inter-rater Spearman rank correlation was high and statistically significant (rho =0.81, P<0.001).
SF-12
The SF-12 is a 12-item generic measure of health status developed from the widely used SF-36 (83). The second version of the SF-12 (SF-12 v2) can yield scores for eight domains: physical functioning, role physical, bodily pain, general health, vitality, social functioning, role emotional, and mental health. It also provides overall summaries of the physical and mental components. After reversal and recalibration, the scores can be transformed to a 0-100 scale and then to norm-based scores, with higher scores indicating better health. The Chinese version of SF-12 v2 was used successfully in a previous study (84).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Parkin DM, Bray F, Ferlay J, et al. Global cancer statistics, 2002. CA Cancer J Clin 2005;55:74-108. [PubMed]
- El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008;134:1752-63. [PubMed]
- Nordenstedt H, White DL, El-Serag HB. The changing pattern of epidemiology in hepatocellular carcinoma. Dig Liver Dis 2010;42 Suppl 3:S206-14. [PubMed]
- Chan AT, Kishi Y, Chan SL, et al. Accomplishments in 2007 in the management of hepatobiliary cancers. Gastrointest Cancer Res 2008;2:S25-31. [PubMed]
- Steel JL, Eton DT, Cella D, et al. Clinically meaningful changes in health-related quality of life in patients diagnosed with hepatobiliary carcinoma. Ann Oncol 2006;17:304-12. [PubMed]
- Edwards BK, Brown ML, Wingo PA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst 2005;97:1407-27. [PubMed]
- Geschwind JF, Ramsey DE, Choti MA, et al. Chemoembolization of hepatocellular carcinoma: results of a metaanalysis. Am J Clin Oncol 2003;26:344-9. [PubMed]
- Lau WY, Lai EC. Hepatocellular carcinoma: current management and recent advances. Hepatobiliary Pancreat Dis Int 2008;7:237-57. [PubMed]
- Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology 2003;37:429-42. [PubMed]
- Cahill BA, Braccia D. Current treatment for hepatocellular carcinoma. Clin J Oncol Nurs 2004;8:393-9. [PubMed]
- Slevin ML. Quality of life: philosophical question or clinical reality? BMJ 1992;305:466-9. [PubMed]
- Mayer RJ. Summary of the Second International Conference on Biology, Prevention and Treatment of Gastrointestinal Malignancies. Ann Oncol 1995;6:645-9. [PubMed]
- Moinpour CM. Measuring quality of life: an emerging science. Semin Oncol 1994;21:48-60; discussion 60-3. [PubMed]
- McNeil BJ, Weichselbaum R, Pauker SG. Speech and survival: tradeoffs between quality and quantity of life in laryngeal cancer. N Engl J Med 1981;305:982-7. [PubMed]
- Qiao CX, Zhai XF, Ling CQ, et al. Health-related quality of life evaluated by tumor node metastasis staging system in patients with hepatocellular carcinoma. World J Gastroenterol 2012;18:2689-94. [PubMed]
- Yeo W, Mo FK, Koh J, et al. Quality of life is predictive of survival in patients with unresectable hepatocellular carcinoma. Ann Oncol 2006;17:1083-9. [PubMed]
- Granda-Cameron C, Viola SR, Lynch MP, et al. Measuring patient-oriented outcomes in palliative care: functionality and quality of life. Clin J Oncol Nurs 2008;12:65-77. [PubMed]
- Fayers P, Bottomley A, EORTC Quality of Life Group, et al. Quality of life research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer 2002;38 Suppl 4:S125-33. [PubMed]
- Poon RT, Fan ST, Yu WC, et al. A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg 2001;136:693-9. [PubMed]
- Tanabe G, Ueno S, Maemura M, et al. Favorable quality of life after repeat hepatic resection for recurrent hepatocellular carcinoma. Hepatogastroenterology 2001;48:506-10. [PubMed]
- Brans B, Lambert B, De Beule E, et al. Quality of life assessment in radionuclide therapy: a feasibility study of the EORTC QLQ-C30 questionnaire in palliative (131)I-lipiodol therapy. Eur J Nucl Med Mol Imaging 2002;29:1374-9. [PubMed]
- Ueno S, Tanabe G, Nuruki K, et al. Quality of life after hepatectomy in patients with hepatocellular carcinoma: implication of change in hepatic protein synthesis. Hepatogastroenterology 2002;49:492-6. [PubMed]
- Zhao JB, Li YH, Chen Y, et al. Evaluation of quality of life before and after interventional therapy in patients with primary hepatocellular carcinoma. Chin J Radiol 2002;36:873-6.
- Bianchi G, Loguercio C, Sgarbi D, et al. Reduced quality of life of patients with hepatocellular carcinoma. Dig Liver Dis 2003;35:46-54. [PubMed]
- Steel J, Baum A, Carr B. Quality of life in patients diagnosed with primary hepatocellular carcinoma: hepatic arterial infusion of Cisplatin versus 90-Yttrium microspheres (Therasphere). Psychooncology 2004;13:73-9. [PubMed]
- Chen L, Liu Y, Li GG, et al. Quality of life in patients with liver cancer after operation: a 2-year follow-up study. Hepatobiliary Pancreat Dis Int 2004;3:530-3. [PubMed]
- Steel JL, Geller DA, Carr BI. Proxy ratings of health related quality of life in patients with hepatocellular carcinoma. Qual Life Res 2005;14:1025-33. [PubMed]
- Wang YB, Chen MH, Yan K, et al. Quality of life of primary hepatocellular carcinoma patients after radiofrequency ablation. Ai Zheng 2005;24:827-33. [PubMed]
- Eid S, Stromberg AJ, Ames S, et al. Assessment of symptom experience in patients undergoing hepatic resection or ablation. Cancer 2006;107:2715-22. [PubMed]
- Lee LJ, Chen CH, Yao G, et al. Quality of life in patients with hepatocellular carcinoma received surgical resection. J Surg Oncol 2007;95:34-9. [PubMed]
- Wang YB, Chen MH, Yan K, et al. Quality of life after radiofrequency ablation combined with transcatheter arterial chemoembolization for hepatocellular carcinoma: comparison with transcatheter arterial chemoembolization alone. Qual Life Res 2007;16:389-97. [PubMed]
- Kondo Y, Yoshida H, Tateishi R, et al. Health-related quality of life of chronic liver disease patients with and without hepatocellular carcinoma. J Gastroenterol Hepatol 2007;22:197-203. [PubMed]
- Steel JL, Chopra K, Olek MC, et al. Health-related quality of life: Hepatocellular carcinoma, chronic liver disease, and the general population. Qual Life Res 2007;16:203-15. [PubMed]
- Martin RC, Eid S, Scoggins CR, et al. Health-related quality of life: return to baseline after major and minor liver resection. Surgery 2007;142:676-84. [PubMed]
- Sun V, Ferrell B, Juarez G, et al. Symptom concerns and quality of life in hepatobiliary cancers. Oncol Nurs Forum 2008;35:E45-52. [PubMed]
- Méndez Romero A, Wunderink W, van Os RM, et al. Quality of life after stereotactic body radiation therapy for primary and metastatic liver tumors. Int J Radiat Oncol Biol Phys 2008;70:1447-52. [PubMed]
- Nowak AK, Cebon J, Hargreaves C, et al. Assessment of health-related quality of life and patient benefit as outcome measures for clinical trials in hepatocellular carcinoma. Asia-Pacific J Clinical Oncology 2008;4:55-67.
- Cebon J, Findlay M, Hargreaves C, et al. Somatostatin receptor expression, tumour response, and quality of life in patients with advanced hepatocellular carcinoma treated with long-acting octreotide. Br J Cancer 2006;95:853-61. [PubMed]
- Bonnetain F, Paoletti X, Collette S, et al. Quality of life as a prognostic factor of overall survival in patients with advanced hepatocellular carcinoma: results from two French clinical trials. Qual Life Res 2008;17:831-43. [PubMed]
- Doffoël M, Bonnetain F, Bouché O, et al. Multicentre randomised phase III trial comparing Tamoxifen alone or with Transarterial Lipiodol Chemoembolisation for unresectable hepatocellular carcinoma in cirrhotic patients (Fédération Francophone de Cancérologie Digestive 9402). Eur J Cancer 2008;44:528-38. [PubMed]
- Barbare JC, Bouché O, Bonnetain F, et al. Randomized controlled trial of tamoxifen in advanced hepatocellular carcinoma. J Clin Oncol 2005;23:4338-46. [PubMed]
- Wible BC, Rilling WS, Drescher P, et al. Longitudinal quality of life assessment of patients with hepatocellular carcinoma after primary transarterial chemoembolization. J Vasc Interv Radiol 2010;21:1024-30. [PubMed]
- Shun SC, Chen CH, Sheu JC, et al. Quality of life and its associated factors in patients with hepatocellular carcinoma receiving one course of transarterial chemoembolization treatment: a longitudinal study. Oncologist 2012;17:732-9. [PubMed]
- Molinari M, Kachura JR, Dixon E, et al. Transarterial chemoembolisation for advanced hepatocellular carcinoma: results from a North American cancer centre. Clin Oncol (R Coll Radiol) 2006;18:684-92. [PubMed]
- Diouf M, Filleron T, Barbare JC, et al. The added value of quality of life (QoL) for prognosis of overall survival in patients with palliative hepatocellular carcinoma. J Hepatol 2013;58:509-21. [PubMed]
- Fan SY, Eiser C, Ho MC, et al. Health-related quality of life in patients with hepatocellular carcinoma: the mediation effects of illness perceptions and coping. Psychooncology 2013;22:1353-60. [PubMed]
- Samonakis DN, Moschandreas J, Arnaoutis T, et al. Treatment of hepatocellular carcinoma with long acting somatostatin analogues. Oncol Rep 2002;9:903-7. [PubMed]
- Dimitroulopoulos D, Xinopoulos D, Tsamakidis K, et al. The role of sandostatin LAR in treating patients with advanced hepatocellular cancer. Hepatogastroenterology 2002;49:1245-50. [PubMed]
- Chow PK, Tai BC, Tan CK, et al. High-dose tamoxifen in the treatment of inoperable hepatocellular carcinoma: A multicenter randomized controlled trial. Hepatology 2002;36:1221-6. [PubMed]
- Yuen MF, Poon RT, Lai CL, et al. A randomized placebo-controlled study of long-acting octreotide for the treatment of advanced hepatocellular carcinoma. Hepatology 2002;36:687-91. [PubMed]
- Poon RT, Yu WC, Fan ST, et al. Long-term oral branched chain amino acids in patients undergoing chemoembolization for hepatocellular carcinoma: a randomized trial. Aliment Pharmacol Ther 2004;19:779-88. [PubMed]
- Steel J, Hess SA, Tunke L, et al. Sexual functioning in patients with hepatocellular carcinoma. Cancer 2005;104:2234-43. [PubMed]
- Kirchhoff TD, Rudolph KL, Layer G, et al. Chemoocclusion vs chemoperfusion for treatment of advanced hepatocellular carcinoma: a randomised trial. Eur J Surg Oncol 2006;32:201-7. [PubMed]
- Verset G, Verslype C, Reynaert H, et al. Efficacy of the combination of long-acting release octreotide and tamoxifen in patients with advanced hepatocellular carcinoma: a randomised multicentre phase III study. Br J Cancer 2007;97:582-8. [PubMed]
- Becker G, Allgaier HP, Olschewski M, et al. Long-acting octreotide versus placebo for treatment of advanced HCC: a randomized controlled double-blind study. Hepatology 2007;45:9-15. [PubMed]
- Dimitroulopoulos D, Xinopoulos D, Tsamakidis K, et al. Long acting octreotide in the treatment of advanced hepatocellular cancer and overexpression of somatostatin receptors: randomized placebo-controlled trial. World J Gastroenterol 2007;13:3164-70. [PubMed]
- Steel JL, Gamblin TC, Carr BI. Measuring post-traumatic growth in people diagnosed with hepatobiliary cancer: directions for future research. Oncol Nurs Forum 2008;35:643-50. [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [PubMed]
- Barbare JC, Bouché O, Bonnetain F, et al. Treatment of advanced hepatocellular carcinoma with long-acting octreotide: a phase III multicentre, randomised, double blind placebo-controlled study. Eur J Cancer 2009;45:1788-97. [PubMed]
- Jia WD, Zhang CH, Xu GL, et al. Octreotide therapy for hepatocellular carcinoma: a systematic review of the evidence from randomized controlled trials. Hepatogastroenterology 2010;57:292-9. [PubMed]
- Kouroumalis E, Skordilis P, Thermos K, et al. Treatment of hepatocellular carcinoma with octreotide: a randomized controlled study. Gut 1998;42:442-7. [PubMed]
- Dollinger MM, Lautenschlaeger C, Lesske J, et al. Thymostimulin versus placebo for palliative treatment of locally advanced or metastasised hepatocellular carcinoma: a phase III clinical trial. BMC Cancer 2010;10:457. [PubMed]
- Chow PK, Machin D, Chen Y, et al. Randomised double-blind trial of megestrol acetate vs placebo in treatment-naive advanced hepatocellular carcinoma. Br J Cancer 2011;105:945-52. [PubMed]
- Soliman H, Ringash J, Jiang H, et al. Phase II trial of palliative radiotherapy for hepatocellular carcinoma and liver metastases. J Clin Oncol 2013;31:3980-6. [PubMed]
- Tuinman MA, Hoekstra HJ, Sleijfer DT, et al. Testicular cancer: a longitudinal pilot study on stress response symptoms and quality of life in couples before and after chemotherapy. Support Care Cancer 2007;15:279-86. [PubMed]
- Szabo S. The World Health Organization Quality of Life (WHOQOL) assessment instrument. In: Spilker B. eds. Quality of life and pharmacoeconomics in clinical trials. Philadelphia PA: Lippincott-Raven;1996:355-62.
- Cella D, Bullinger M, Scott C, et al. Group vs individual approaches to understanding the clinical significance of differences or changes in quality of life. Mayo Clin Proc 2002;77:384-92. [PubMed]
- Guyatt GH, Osoba D, Wu AW, et al. Methods to explain the clinical significance of health status measures. Mayo Clin Proc 2002;77:371-83. [PubMed]
- Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365-76. [PubMed]
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9. [PubMed]
- Cella D, Yount S, Sorensen M, et al. Validation of the Functional Assessment of Chronic Illness Therapy Fatigue Scale relative to other instrumentation in patients with rheumatoid arthritis. J Rheumatol 2005;32:811-9. [PubMed]
- Fayers PM, Aaronson NK, Bjordal K, et al. On behalf of the EORTC quality of life group. The EORTC QLQ-C30 scoring manual (3rd edition). Brussels: European Organization for Research and Treatment of Cancer, 2001.
- Blazeby JM, Currie E, Zee BC, et al. Development of a questionnaire module to supplement the EORTC QLQ-C30 to assess quality of life in patients with hepatocellular carcinoma, the EORTC QLQ-HCC18. Eur J Cancer 2004;40:2439-44. [PubMed]
- Heffernan N, Cella D, Webster K, et al. Measuring health-related quality of life in patients with hepatobiliary cancers: the functional assessment of cancer therapy-hepatobiliary questionnaire. J Clin Oncol 2002;20:2229-39. [PubMed]
- Yount S, Cella D, Webster K, et al. Assessment of patient-reported clinical outcome in pancreatic and other hepatobiliary cancers: the FACT Hepatobiliary Symptom Index. J Pain Symptom Manage 2002;24:32-44. [PubMed]
- Montazeri A, Goshtasebi A, Vahdaninia M, et al. The Short Form Health Survey (SF-36): translation and validation study of the Iranian version. Qual Life Res 2005;14:875-82. [PubMed]
- Apolone G, Filiberti A, Cifani S, et al. Evaluation of the EORTC QLQ-C30 questionnaire: a comparison with SF-36 Health Survey in a cohort of Italian long-survival cancer patients. Ann Oncol 1998;9:549-57. [PubMed]
- World Health Organization’s Quality of Life group Measuring Quality of Life; Development of the World Health Organization Quality of Life Instrument (WHOQOL) 1992.
- World Health Organization’s Quality of Life group WHOQOL-BREF Introduction, Administration and Scoring, Field Trial version. 1996.
- Huang IC, Wu AW, Frangakis C. Do the SF-36 and WHOQOL-BREF measure the same constructs? Evidence from the Taiwan population*. Qual Life Res 2006;15:15-24. [PubMed]
- Addington-Hall JM, MacDonald LD, Anderson HR. Can the Spitzer Quality of Life Index help to reduce prognostic uncertainty in terminal care? Br J Cancer 1990;62:695-9. [PubMed]
- Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. [PubMed]
- Lam CL, Tse EY, Gandek B. Is the standard SF-12 health survey valid and equivalent for a Chinese population? Qual Life Res 2005;14:539-47. [PubMed]


