Pre-operative percutaneous endoscopic gastrostomy tube placement does not increase post-operative complications or mortality in oesophageal cancer
Introduction
Patients with oesophageal cancer often present with dysphagia and weight loss. The resulting malnutrition has a significant impact on subsequent management of these patients. Percutaneous endoscopic gastrostomy (PEG) tubes have been employed as a useful adjunct in fulfilling the nutritional requirements of these patients (1,2). Many authors, however, continue to caution against the placement of PEG tubes in patients with oesophageal cancer, owing to a potential risk of tumour seeding at the PEG site, perceived difficulty in using the stomach as a substitute for the oesophagus following oesophagectomy and fear of an increased risk of post-operative complications, including anastomotic leak (3,4).
Some studies, however, suggest that PEG tubes in oesophageal cancer are safe, useful and do not compromise the stomach or the oesophago-gastric anastomosis (5-7).
An average of 150 to 200 new patients with oesophageal cancer present to our institution each year and PEG tubes are inserted in more than 95% of these before commencement of neo-adjuvant chemoradiotherapy. PEG tubes are placed by Ponsky’s pull technique (8). In order to address these contradictory views, we felt it would be useful to share our experience by performing a retrospective cross-sectional review of oesophageal cancer patients in whom a PEG tube was placed prior to initiation of neo-adjuvant treatment.
Objective
(I) To assess the impact of PEG tubes on nutritional status, multimodality treatment and post-operative complications in patients with oesophageal cancer who underwent PEG tube insertion prior to treatment; (II) to assess overall survival at 3 years after treatment.
Methods
The study synopsis was reviewed by the Institutional Review Board (IRB) of the Shaukat Khanum Cancer Hospital & Research Center, Lahore (IRB00005898). After review, IRB granted exemption as the study involved the data, which was existing in the medical records (retrospective review) and it was recorded without identifiers of the participants (IRB number: 15-03-18-01). IRB also granted a waiver of informed consent as the research involved no direct patient contact, no risk to subjects and that the waiver will not adversely affect the rights and welfare of the subjects.
Study design
Retrospective cross-sectional study.
Setting
Shaukat Khanum Memorial Cancer Hospital & Research Centre, Lahore (SKMCH&RC), a tertiary care cancer hospital in Pakistan.
Inclusion criteria
All patients with oesophageal or gastro oesophageal junction (GOJ) cancer, who underwent PEG tube insertion and for whom neo-adjuvant treatment, followed by surgery was planned in a multi-disciplinary team meeting (MDT).
Data collection
Data was collected retrospectively from our institution’s electronic database, for 800 patients who presented with oesophageal or GOJ cancer over the five-year period from June 01, 2010 to May 31, 2015. A total of 168 patients, who met the inclusion criteria, were analysed further. Data of all patients was reviewed for the three-year period following completion of treatment to calculate the overall survival. Thus, the follow up of the last patient, included in the study was completed on May, 31, 2018.
Baseline characteristics, including demographic details, gender, age, body mass index (BMI), primary diagnoses, histopathology as well as serum albumin were recorded. Assessment of nutritional status was done by recording average body mass index and serum albumin levels prior to PEG tube insertion and then before surgery. Complications arising from PEG tube insertion, and 1-month mortality rates were calculated. The impact of the PEG tube on surgery, including delay in surgery or difficulty with gastric conduit formation, as well as postoperative complications, was recorded.
Statistical analysis
Statistical analysis was carried out using the Statistical Package for the Social Sciences (SPSS) software (version 20.0; SPSS, Chicago, IL, USA). Descriptive analysis was done using summary measures for categorical variables and continuous variables. Categorical variables were computed as frequencies and percentages, while continuous variables were stated as mean ± standard deviation. Overall survival was calculated using Kaplan-Meier curves. A P value of ≤0.05 was considered statistically significant.
Results
General characteristics & nutritional status
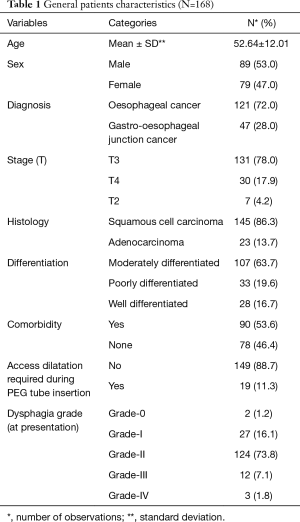
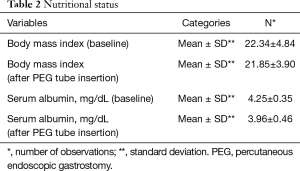
Out of 168 patients, 89 (53.0%) were male. The mean age of our patients was 52.64±12.01. Most of our patients had grade-II dysphagia (124/168, 73.8%), as shown in Table 1. Furthermore, the majority of our patients had T3 tumours (78.0%), with squamous cell carcinoma being the predominant histology (86.3%). Ninety out of 168 (53.6%) had co-morbidities, including diabetes mellitus, hypertension and ischaemic heart disease, as shown in Table 1. The mean BMI at presentation was 22.34, while mean serum albumin level was 4.25 mg/dL, as shown in Table 2. All of these patients were seen by a qualified nutritionist on the day of PEG tube insertion and given a formal diet plan for feeding with their PEG tube. The mean BMI and serum albumin after PEG tube insertion and neo-adjuvant treatment were 21.85 and 3.96 mg/dL respectively, which were calculated before surgery, as shown in Table 2. Thirty-three out of 168 patients (19.6%) suffered a complication as a result of PEG tube insertion, as shown in Table 3. Infection was the most common complication (24/168, and 14.3%) and was treated with antibiotics, usually via the PEG tube. PEG-related mortality at 1 month was 0%.
Full table
Full table
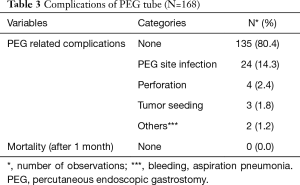
Full table
Impact of PEG tube on nutritional status
The average BMI of the patients was maintained throughout the course of neo-adjuvant treatment (22.34±4.84 before PEG vs. 21.85±3.90 after PEG, P value: 0.1). The decline in serum albumin was statistically significant. However, the mean value remained above 3.5 mg/dL (4.25±0.35 before PEG vs. 3.96±0.46 after PEG, P value: 0.0). Moreover, in only 5/69 (7.2%) patients, surgery was deferred due to poor nutritional status despite the presence of PEG tube.
Impact of PEG tube on surgery
Surgery was successfully performed, after neo-adjuvant treatment, in 99/168 (58.9%) patients, as shown in Table 4. Gastric conduit formation was possible in all 99 patients, who underwent surgery. Surgery was deferred in 3/168 (1.8%) patients due to PEG site tumour seeding, while 5/168 (3.0%) could not have it done due to poor nutritional status despite the PEG tube insertion. The other reasons for deferring surgery are shown in Table 5.
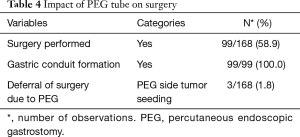
Full table
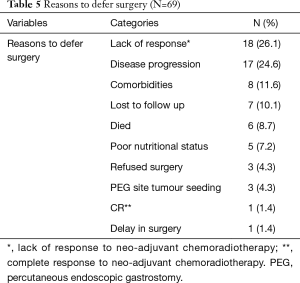
Full table
Post-operative complications
Post-operative complications (both immediate and late) were seen in 17/99 patients (17.17%), as shown in Table 6, with surgical site infection being the most common (7.07%).

Full table
Survival at 3 years of follow up
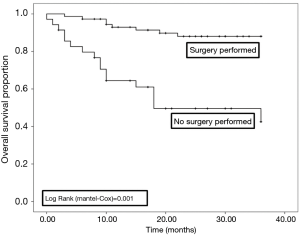
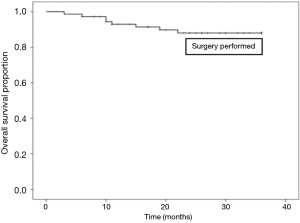
Sixty out of 168 patients were lost to follow up as shown in Table 7. Overall survival at 3 years was 76.8% in all patients. There was a statistically significant difference in survival between those who did (87.0%), and those who did not have surgery (50.0%), as shown in Figures 1,2.
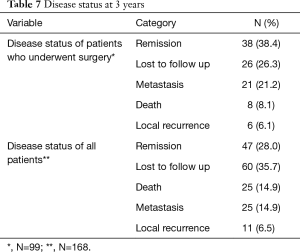
Full table
Discussion
The role of PEG tube insertion as part of the treatment process is well established in patients with head and neck cancer. These patients are prone to develop malnutrition because of dysphagia secondary to disease burden and radiation associated side effects (1,2). The concept of nutritional bridging during neo-adjuvant treatment is also important in patients with oesophageal cancer (9-12), and this seems to be supported by our results, which reveal that PEG feeding allowed maintenance of the average BMI of patients during neo-adjuvant treatment. This is particularly important because neo-adjuvant treatment is widely recommended and used, as it has shown to improve survival in operable esophageal cancer (23% vs. 17%) and should be considered as a standard of care (13). Moreover, in our part of the world, patients frequently present at an advanced stage of cancer, with significant dysphagia and often with weight loss, which necessitates the role of PEG feeding. However, PEG tube insertion in such patients has not been widely recommended due to a perceived risk of tumour implantation at the PEG tube insertion site, as well as fear that the stomach may no longer be suitable for conduit formation and that the risk of post-operative complications, particularly anastomotic leak, may increase (3). On the other hand, there are a few studies which support the use of PEG tubes prior to neo-adjuvant treatment and surgery for oesophageal cancer. Stockeld et al. reported in a retrospective analysis of 229 patients that out of 222 patients who had pre-operative PEG tube insertion, only 1 (0.9%) died due to perforation, 1 patient had tumour implantation at the PEG site (0.9%) and 2 (1.8%) had an anastomotic leak (6). The study was limited by the fact that it did not have a control group in whom a pre-operative PEG tube was not inserted. Wright et al. compared two cohorts of patients with and without pre-operative PEG tube insertion. Their results were not against the use of PEG tube, since their rate of gastric conduit formation was similar between the two groups (94% in patients with pre-operative PEG vs. 87% without PEG, P=0.27). Anastomotic leak rates were likewise similar in the two groups (11% in patients with pre-operative PEG vs. 15% without PEG, P=0.65) (7).
Our results confirm that PEG tubes are safe prior to neo-adjuvant treatment. Gastric conduit formation was possible in all our patients and the post-operative complication rate was no different to those patients who underwent surgery without prior PEG tube insertion, as reported in the literature. For instance, the most common reason to avoid a PEG tube in these patients is the risk of anastomotic leak. A recent study showed that anastomotic leaks following oesophagectomy without pre-operative PEG tube occur in up to 19.4% of patients, while in another study, this rate was reported as 17.2% (14,15). In our study, anastomotic leaks were seen in only 6% of patients, suggesting that, at least in our cohort, PEG tube insertion does not increase the risk of anastomotic leak. Another late complication which is thought to be more common following PEG tube insertion is anastomotic site stricture. The available literature suggests this to occur in between 1–9% without pre-operative PEG tube (16,17). Only 4% of the patients in our cohort developed an anastomotic site stricture.
At our institute, we perform PEG in nearly all newly diagnosed patients with oesophageal cancer and feedback from our surgical colleagues is that PEG cause minimal adhesions as compared with radiological or surgical gastrostomy. The location of gastrostomy is important, as long as it is fashioned at the anterior wall of stomach away from both greater and lesser curvature; it causes minimal problems during conduit formation. If the PEG site is closed longitudinally it causes minimal disruption to the submucosal blood vessels which are imperative for the viability of gastric conduit. PEG has added advantage, if the gastric reconstruction is performed laparoscopically. It helps to lift the anterior wall of the stomach and facilitates entry to the lesser sac and gastric mobilisation.
The overall mortality in oesophageal cancer patients without PEG tube, undergoing oesophagectomy is reported to be 8% in one study and 12% in another study (18,19), which is comparable to 8.1% in our study. We report the long-term overall survival at 3 years of follow up as 76%, which has been shown as 48.2% to 79.5% in a study, depending upon lymphatic spread (20).
Conclusions
Pre-operative PEG tube insertion in patients with oesophageal cancer is safe and does not compromise subsequent surgery in any way. In addition, it is helpful in maintaining the nutritional requirements of these patients with dysphagia during neo-adjuvant treatment, with excellent outcomes seen at 3 years of follow up.
Our study was limited by the fact that it was a retrospective review and did not have a control group of patients who underwent surgery without having a PEG tube inserted.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study synopsis was reviewed by the Institutional Review Board (IRB) of the Shaukat Khanum Cancer Hospital & Research Center, Lahore (IRB00005898). IRB granted exemption (IRB number: 15-03-18-01) and a waiver of informed consent.
References
- Role of PEG/PEJ in enteral feeding. American Society for Gastrointestinal Endoscopy. Gastrointest Endosc 1998;48:699-701. [Crossref] [PubMed]
- Marcy PY, Magné N, Bensadrum RJ, et al. Percutaneous endoscopic gastrostomy: lost/benefit analysis in patients with carcinoma of the upper aero-digestive tract. Bull Cancer 2000;87:329-33. [PubMed]
- Pickhardt PJ, Rohrmann CA Jr, Cossentino MJ. Stomal metastases complicating percutaneous endoscopic gastrostomy: CT findings and the argument for radiologic tube placement. AJR Am J Roentgenol 2002;179:735-9. [Crossref] [PubMed]
- Schrag SP, Sharma R, Jaik NP, et al. Complications related to percutaneous endoscopic gastrostomy (PEG) tubes. A comprehensive clinical review. J Gastrointestin Liver Dis 2007;16:407-18. [PubMed]
- Margolis M, Alexander P, Trachiotis GD, et al. Percutaneous endoscopic gastrostomy before multimodality therapy in patients with esophageal cancer. Ann Thorac Surg 2003;76:1694-7; discussion 1697-8.
- Stockeld D, Fagerberg J, Granström L, et al. Percutaneous endoscopic gastrostomy for nutrition in patients with oesophageal cancer. Eur J Surg 2001;167:839-44. [Crossref] [PubMed]
- Wright GP, Foster SM, Chung MH. Esophagectomy in patients with prior percutaneous endoscopic gastrostomy tube placement. Am J Surg 2014;207:361-5; discussion 364-5. [Crossref] [PubMed]
- Gauderer MW, Ponsky JL, Izant RJ Jr. Gastrostomy without laparotomy: a percutaneous endoscopic technique. 1980. Nutrition 1998;14:736-8. [PubMed]
- Fan ST, Lau WY, Wong KK, et al. Pre-operative parenteral nutrition in patients with oesophageal cancer: a prospective, randomised clinical trial. Clin Nutr 1989;8:23-7. [Crossref] [PubMed]
- Moghissi K, Hovashaw J, Teasdale PR, et al. Parenteral nutrition in carcinoma of the esophagus treated by surgery: nitrogen balance and clinical studies. Br J Surg 1977;64:125-8. [Crossref] [PubMed]
- Dewitt RC, Kurdsk KA. Enteral nutrition. Gastroenterol Clin North Am 1998;27:371-86. [Crossref] [PubMed]
- Lipman TO. Grains or veins: is enteral nutrition really better than parenteral nutrition? A look at the evidence. JPEN J Parenter Enteral Nutr 1998;22:167-82. [Crossref] [PubMed]
- Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol 2009;27:5062-7. [Crossref] [PubMed]
- Booka E, Takeuchi H, Nishi T, et al. The impact of postoperative complications on survivals after esophagectomy for esophageal cancer. Medicine (Baltimore) 2015;94:e1369. [Crossref] [PubMed]
- Saluja SS, Ray S, Pal S, et al. Randomized trial comparing side-to-side stapled and hand-sewn esophagogastric anastomosis in neck. J Gastrointest Surg 2012;16:1287-95. [Crossref] [PubMed]
- Chen KN. Managing complications I: leaks, strictures, emptying, reflux, chylothorax. J Thorac Dis 2014;6 Suppl 3:S355-63. [PubMed]
- Law S, Fok M, Chu KM, et al. Comparison of hand-sewn and stapled esophagogastric anastomosis after esophageal resection for cancer: a prospective randomized controlled trial. Ann Surg 1997;226:169-73. [Crossref] [PubMed]
- Jamieson GG, Mathew G, Ludemann R, et al. Postoperative mortality following oesophagectomy and problems in reporting its rate. Br J Surg 2004;91:943-7. [Crossref] [PubMed]
- McCulloch P, Ward J, Tekkis PP, et al. Mortality and morbidity in gastro-oesophageal cancer surgery: initial results of ASCOT multicentre prospective cohort study. BMJ 2003;327:1192-7. [Crossref] [PubMed]
- Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 573-5. [PubMed]