Rectal cancer in the young: analysis of contributing factors and surgical outcomes
Introduction
Rectal cancer (RC) among younger patients (≤50 years) is on the rise in the United States (1). While there has been a steady decline in incidence rates of RC for patients 50 years and older, the opposite trend is observed in younger patients (2). The observed decline in incidence for older patient is attributed to the improvements in clinical guidelines, screening, and early removal of adenomatous polyps if noted (3). The increased incidence of RC in younger patients is most significant for patients 20–34 years for localized, regional and metastasized disease (2,4). In addition, younger patients are more likely to present with stages III–IV disease and disease with more aggressive features (mucinous and signet ring cell) than patients 50 years and older (3,4)
The factors associated with development of RC are well established. However, factors leading to early onset RC (≤50 years) remain unclear and warrant further study. Predisposing syndromes such as hereditary nonpolyposis colorectal cancer (HNPCC) and recognized pathways of RC carcinogenesis such as APC-mutation have been linked to a small percentage of RC in patient’s ≤50 years of age (5,6). Disparities in incidence of RC have also been established. The CONCORD 2 study highlighted a narrowing in the racial disparities that existed in the 5-year survival rates of RC; however, the 5-year overall survival rate in the black population remains lower compared to other races. Disparity also exists based on geographical location, with rural settings having poorer survival outcomes (7). The commonality between both disparities is a lower socioeconomic status and access to adequate healthcare. Obesity is another contributing factor in the prevalence of early onset RC (8). In a recent population based study analyzing a cohort of 1.8 million adults followed over 23 years, obesity, defined as a BMI greater than 29.6 in men, and 30.6 in women, was associated with a near 2 fold increase in the risk for RC (for men, HR 1.71; 95% CI, 1.11–2.65; significant from a BMI of 29.6 kg/m2; for women, HR 2.03; 95% CI, 0.90–4.58; significant from a BMI of 30.6 kg/m2) (9).
Modifiable risk factors such as obesity, alcohol, smoking (10,11) has been associated with an increased risk of developing certain tumors; identifying such modifiable and non-modifiable risk factors (race, gender) in RC (12) will help direct early screening efforts to at risk populations, ultimately leading to intervention in the early stages. Early detection correlates with early intervention that typically results in reduction of incidence, morbidity and mortality of disease (13). Understanding the factors associated with early onset RC in younger patients could help decelerate the current rise in incidence of RC in patients <50 years of age. The aim of this study was to address the alarming trend in the increased incidence of RC in younger patients by assessing the factors that are associated with RC in this group of patients. We will also achieve this by comparing the comorbidities and clinical presentations between the younger and older RC patients.
Methods
Data source
This is a 3-year [2010–2012] retrospective analysis of the National Inpatient Sample (NIS) database, which is maintained by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project (HCUP). The NIS databank is the largest all-payer in-patient care database publically available in the United States. It covers 95% of the US population and includes comprehensive abstracted data. The data in the NIS are derived from a stratified sample of 20% of the discharges from all community hospitals (short-term, non-federal, general, specialty, and non-rehabilitation hospitals) in the US. The data are weighted back to help make population estimates of the various parameters. For our study, the use of the NIS database was conformed to the data-use agreement from the HCUP. Every year, the NIS contains information for nearly 8 million weighted discharges from over 1,100 hospitals across 44 states in the United States.
Patient population
Patients with RC were identified from the NIS database using the Ninth Revision of the International Classification of Diseases diagnosis codes for RC (ICD-9 CM diagnosis code 48). We excluded colon cancer patients, transferred patients and missing data were excluded.
Data points collected
We abstracted data on patients’ demographics (age, race, and gender), insurance status, teaching status of the hospital, location of the hospital (urban vs. rural), operative approach, severity of illness, complications, hospital length of stay.
Outcome measure
The outcome of our study was to assess the differences in demographics, presenting symptoms and comorbidities. In addition, we assessed differences in in-hospital complications, hospital length of stay, in-hospital mortality. We defined complications as urinary tract infections (ICD-9 CM diagnosis code 599.0), sepsis (ICD-9 CM diagnosis codes 995.91 and 995.92), pneumonia (ICD-9 CM diagnosis codes 480.0–480.9, 481, 482.0–482.9, 483.0–483.8, 485–487, and 507), deep venous thrombosis and pulmonary embolism (ICD-9 CM diagnosis codes 453.40–453.42, and 415.1), respiratory dysfunction (ICD-9 CM diagnosis codes 518.51–518.53 and 518.81), renal (ICD-9 CM diagnosis codes 584.5–584.9 and 593.9), cardiac/cerebrovascular (ICD-9 CM diagnosis codes 410, 427.5, and 434.91). Surgery related complications includes surgical site infection (ICD-9 CM diagnosis codes 998.5, 998.51, and 998.59) and re-operation, disruption of surgical wound (ICD-9 CM diagnosis codes 998.3, and 998.31–998.32), and abdominal abscess (ICD-9 CM diagnosis code 567.22).
Statistical analysis
Data are reported as the mean ± standard deviation (SD) for continuous descriptive variables, as the median [range] for ordinal descriptive variables and as the proportion for categorical variables. We used the Mann-Whitney U test and the Student t-test to explore for differences in the two groups (young and old) for continuous variables. We utilized Chi-square test to identify differences in outcomes between the two groups for categorical variables. Regression analysis (logistic regression for complications and mortality and log-linear regression for hospital LOS) was performed to compare the two groups while controlling for patient’s characteristics. For our study, we considered P value ≤0.05 as statistically significant. All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, Version 20; SPSS, Inc., Chicago, IL, USA). This study was reviewed by the University of Arizona, Institutional Review Board and was determined to be exempt from the need for approval.
Results
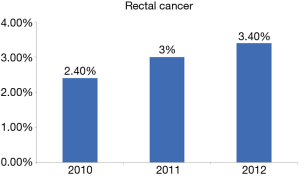
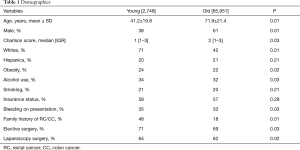
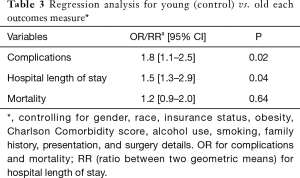
A total of 68,699 patients with RC were included. Mean age was 65.3±15.0 of which 4% were young. Incidence of RC among younger patients increased significantly over the study period (2.8% vs. 3.4%; P=0.04). Figure 1 demonstrates the incidence of rectal cancer over the study period. Majority of the younger patients with RC were white female. Bleeding was the most common presentation amongst younger patients (P=0.03). Younger patients were more likely to have a family history of RC (P=0.01) and were more likely to undergo elective surgery (P=0.04) and laparoscopic surgery (P=0.02) compared to the older patients. Younger patients with RC were more likely to use alcohol (P=0.03) and be obese (P=0.02) compared to older patients. There was no difference in other co-morbidities between the two groups. Tables 1,2 demonstrate the demographic and in-hospital outcomes among the study population. After controlling for all factors in a regression model, younger patients had a lower complication rate (P=0.01), hospital LOS (P=0.02), and mortality rate (P=0.04). Table 3 demonstrates the regression analysis for outcomes among study population.
Full table

Full table
Full table
Discussion
The overall incidence rates for RC in the United States is on the decline; largely attributed to the increase in screening amongst individuals over 50 years of age. However, recent trends have shown a rising incidence of RC amongst younger patients below the age of 50 (14). In our study, we established some of the factors that are attributed to this new trend. Our 3-year retrospective analysis of the National Inpatient Sample (NIS) database evaluated 68,699 patients with RC of which 4% were below the age of 50, with a 1% increase in incidence in the younger population from 2.4% to 3.4% over the 3-year period of this study. This increase in the <50 age group is concerning, especially with a steady decline in the older age group over the last decade (1,15). While there were some overall similar risk factors between both groups such as obesity, alcohol use, and smoking, the younger population showed a significant difference in race and sex. Our findings are consistent with prior studies (7,14) that discuss the trends of RC amongst young patients, but in this study, we highlight the relationships that could provide more insight into the contributory factors of RC in younger patients.
In our study, we demonstrated that the older population with RC were predominantly male, 61%; however, the younger population show a 62% female predominance. This stark contrast in the number of women in the younger age group portends the possibility of a confounding factor that might be unique to this subgroup. Prior studies have shown a positive association between obesity and the risk of colorectal cancer in premenopausal women, with no association and even a mild risk reduction in postmenopausal women (8). This is a possible explanation why, with the increased incidence in younger patients, younger pre-menopausal women have a higher risk. High adiposity in obese patients results in increased insulin levels which in turn increases insulin-like growth factor 1 (IGF-1) (16). IGF-1 is associated with a higher risk of colorectal cancer in both men and women (17,18). Although obesity has long been associated with increased risk of RC overall (9,19), this new data reveals the rates are significantly higher in younger age groups, which is especially important with the marked increase in obesity over the last three decades in the United States (20). Diet modification and reduction in obesity might help slow down this trend.
Our data shows an overall increase in the incidence of RC in white females compared to other sub-groups, with percentages of 62% and 71% both women and white respectively. Bailey et al. showed that the implications of screening in the high-risk populations might have exposed a problem that was already there (2). The screening guidelines were designed to screen older adults over the age of 50 (13) for pre-cancerous polyps; and this system worked well to identify and prevent these lesions from developing into full blown cancer. However, the recent increase of RC amongst younger adults might in part be due to the residual effect of routine screening within the high-risk group. The increase in routine screening and the consequent decrease in incidence might have exposed a relative increase in data points within the younger population that are not routinely screened. This suggests that a more robust screening guideline might be beneficial in identifying these pre-cancerous lesions in younger patients as well.
Multiple studies have shown the impact and importance of environmental factors in the development of colon and RC (21,22). Some of the environmental factors include obesity, dietary style, alcohol consumption and smoking. In our study, we demonstrated that younger patients who developed RC were more likely obese and drank alcohol. We did not find any significant difference between the two groups for smoking. Drinking about 4 glasses of alcohol/per day increased the risk of cancer about 50%. The finding of our study was consistent with that literature and demonstrated higher incidence of cancer in males compared to females. It is very difficult to make a case for screening for asymptomatic patients even if there are significant predisposing factor. The economic impact and risk benefit ratio of screening for RC, even in this vulnerable group, is still questionable and needs further investigation.
Other behavioral factors considered were alcohol use and smoking. Our study showed marginal differences between the two groups with 34% use of alcohol reported in the young group compared to 32% in the older population, and 21% in the young reported smoking versus 20% in the older group. These behaviors have been considered before in work done by Siegel et al.; and there is inadequate evidence to explain the trend (14). There was no significant difference in co-morbidities between the two groups, with both younger and older group showing Charlson score of 1 [1–3] and 2 [1–3] respectively. After controlling for all factors in a regression model; younger patients had a lower complication rate, hospital length of stay, and mortality rate.
Several studies (23,24) have shown genetic factors which are considered non-modifiable risk factors for colo-RC. It has been established that these patients have a higher risk of developing cancer and current screening strategies include guidelines for early screening and identification of these patients to help prevent development of cancer. In our study we did not specifically look into these factors as we believe that extensive evidence is present in this regards and furthermore as these are non-modifiable. Our focus was more to assess modifiable difference between younger and old patient who develop RC and try to provide evidence to help develop prevention strategies.
This study has an inherent limitation because it is a retrospective study of data collected from NIS (national inpatient sample) of patients from their discharge summaries. The discharge summaries are only as good as who has dictated them and many important risk factors may be missed that may contribute to the increased incidence of RC in younger patients. Another limitation was the duration of this study. Although we showed clear differences between both groups, a longer duration with more years of data would have been beneficial to the power of the study. Lastly, many studies have linked HPV infection to colorectal adenocarcinoma although the exact role HPV plays in RC is unknown (25,26). Evaluating HPV infection as a possible risk factor for RC in younger patients would have added strength and innovation to this study, unfortunately, that data was not available in the NIS database.
Conclusions
Despite the limitations of this study, we showed the variations in presentation, demographics and comorbidities that exits among younger and older patients with RC. Race and gender also play a role in the incidence of RC in the young. Identifying these risk factors and having a better understanding of the contribution of the bio-chemical, environmental and genetic factors to this increased incidence, will lead to a more robust intervention plan to help improve care among younger patients with RC.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was reviewed by the University of Arizona, Institutional Review Board and was determined to be exempt from the need for approval.
References
- Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017. [Crossref] [PubMed]
- Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg 2015;150:17-22. [Crossref] [PubMed]
- You YN, Dozois EJ, Boardman LA, et al. Young-onset rectal cancer: presentation, pattern of care and long-term oncologic outcomes compared to a matched older-onset cohort. Ann Surg Oncol 2011;18:2469-76. [Crossref] [PubMed]
- Yeo H, Betel D, Abelson JS, et al. Early-onset Colorectal Cancer is Distinct From Traditional Colorectal Cancer. Clin Colorectal Cancer 2017;16:293-9.e6. [Crossref] [PubMed]
- Pearlman R, Frankel WL, Swanson B, et al. Prevalence and Spectrum of Germline Cancer Susceptibility Gene Mutations Among Patients With Early-Onset Colorectal Cancer. JAMA Oncol 2017;3:464-71. [Crossref] [PubMed]
- Wells K, Wise PE. Hereditary Colorectal Cancer Syndromes. Surg Clin North Am 2017;97:605-25. [Crossref] [PubMed]
- Joseph DA, Johnson CJ, White A, et al. Rectal cancer survival in the United States by race and stage, 2001 to 2009: Findings from the CONCORD-2 study. Cancer 2017;123 Suppl 24:5037-58. [Crossref] [PubMed]
- Terry PD, Miller AB, Rohan TE. Obesity and colorectal cancer risk in women. Gut 2002;51:191-4. [Crossref] [PubMed]
- Levi Z, Kark JD, Katz LH, et al. Adolescent body mass index and risk of colon and rectal cancer in a cohort of 1.79 million Israeli men and women: A population-based study. Cancer 2017;123:4022-30. [Crossref] [PubMed]
- Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA 2008;300:2765-78. [Crossref] [PubMed]
- Cavestro GM, Mannucci A, Zuppardo RA, et al. Early onset sporadic colorectal cancer: Worrisome trends and oncogenic features. Dig Liver Dis 2018;50:521-32. [Crossref] [PubMed]
- Cress RD, Morris C, Ellison GL, et al. Secular changes in colorectal cancer incidence by subsite, stage at diagnosis, and race/ethnicity, 1992-2001. Cancer 2006;107:1142-52. [Crossref] [PubMed]
- Rutter CM, Greenlee RT, Johnson E, et al. Prevalence of colonoscopy before age 50. Prev Med 2015;72:126-9. [Crossref] [PubMed]
- Siegel RL, Jemal A, Ward EM. Increase in incidence of colorectal cancer among young men and women in the United States. Cancer Epidemiol Biomarkers Prev 2009;18:1695-8. [Crossref] [PubMed]
- O'Connell JB, Maggard MA, Liu JH, et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg 2003;69:866-72. [PubMed]
- Powell DR, Suwanichkul A, Cubbage ML, et al. Insulin inhibits transcription of the human gene for insulin-like growth factor-binding protein-1. J Biol Chem 1991;266:18868-76. [PubMed]
- Ma J, Giovannucci E, Pollak M, et al. A prospective study of plasma C-peptide and colorectal cancer risk in men. J Natl Cancer Inst 2004;96:546-53. [Crossref] [PubMed]
- Giovannucci E, Pollak MN, Platz EA, et al. A prospective study of plasma insulin-like growth factor-1 and binding protein-3 and risk of colorectal neoplasia in women. Cancer Epidemiol Biomarkers Prev 2000;9:345-9. [PubMed]
- Liu PH, Wu K, Ng K, et al. Association of Obesity With Risk of Early-Onset Colorectal Cancer Among Women. JAMA Oncol 2019;5:37-44. [Crossref] [PubMed]
- Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999-2004. JAMA 2006;295:1549-55. [Crossref] [PubMed]
- Marley AR, Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet 2016;7:105-14. [PubMed]
- Johnson CM, Wei C, Ensor JE, et al. Meta-analyses of colorectal cancer risk factors. Cancer Causes Control 2013;24:1207-22. [Crossref] [PubMed]
- Lynch HT, Lynch JF, Attard TA. Diagnosis and management of hereditary colorectal cancer syndromes: Lynch syndrome as a model. CMAJ 2009;181:273-80. [Crossref] [PubMed]
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology 2010;138:2044-58. [Crossref] [PubMed]
- Pérez LO, Abba MC, Laguens RM, et al. Analysis of adenocarcinoma of the colon and rectum: detection of human papillomavirus (HPV) DNA by polymerase chain reaction. Colorectal Dis 2005;7:492-5. [Crossref] [PubMed]
- Bodaghi S, Yamanegi K, Xiao SY, et al. Colorectal papillomavirus infection in patients with colorectal cancer. Clin Cancer Res 2005;11:2862-7. [Crossref] [PubMed]