Incidence and prognostic impact of high-risk HPV tumor infection in cervical esophageal carcinoma
Introduction
Cervical esophageal carcinoma (CEC) is an uncommon malignancy (1). Few studies have independently examined CEC patients owing to their rarity, often grouping these patients with hypopharyngeal or more distal esophageal tumors. In spite of this scarcity, the medical literature supports the use of definitive chemoradiotherapy (CRT) for these lesions (2-5), providing similar survival rates to primary surgery without the associated morbidity and mortality of concomitant laryngeal and esophageal resection with reconstruction (6,7).
While contemporary studies have demonstrated the positive prognostic impact of human papillomavirus (HPV) infection in oropharyngeal cancer patients, few studies have examined the impact of HPV infection in esophageal carcinoma (8). HPV infection appears to increase the risk of esophageal squamous cell carcinoma (SCC) (9), although some evidence suggests that HPV infection in esophageal tumors is uncommon and may not correlate with anatomical location within the esophagus (i.e., upper versus lower esophagus) (10). To our knowledge, no study has specifically examined the association of cervical esophagus to HPV status, where infection risk may be higher than distal esophageal cancer lesions. Similarly, no study has examined the prognostic importance of tumor HPV infection on long-term outcomes in esophageal cancer at any tumor location. This study focuses on long-term outcomes for patients treated with definitive CRT for CEC, the incidence of tumor HPV status in CEC, and its potential impact on patient outcomes.
Materials and methods
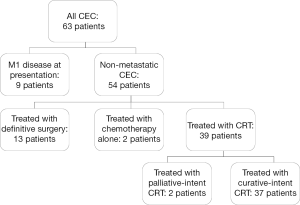
Institutional Review Board (IRB) approval was obtained to identify patients with carcinoma of the cervical esophagus treated with curative-intent CRT at Duke University Medical Center (DUMC) between 1987 and 2013. Radiographic and endoscopic data were independently reviewed by two authors (EBL and BGC) to select those patients with tumors whose epicenter was in the cervical esophagus (between the upper esophageal sphincter and the thoracic inlet). Consensus identified 63 patients with CEC (Figure 1). Of these, eight patients (12.7%) presented with metastatic (M1) disease at diagnosis and were excluded from this study; similarly excluded was one patient (1.6%) who presented with a synchronous metastatic malignancy from a primary breast adenocarcinoma. Also excluded were 13 patients (20.6%) who underwent definitive surgical treatment (esophagectomy), and 2 patients (3.2%) who were treated with palliative chemotherapy alone. Of the 39 patients (61.9%) treated with CRT, 2 patients (3.2%) were treated with palliative intent and were excluded, leaving 37 patients (58.7%) treated with curative-intent CRT included in this study (Figure 1).
Lifetime tobacco abuse was defined by greater than or equal to 10 pack-year of cigarette smoking, or significant lifetime non-cigarette tobacco abuse (>10 years of daily use). Lifetime alcohol abuse was defined as greater than seven drinks per week for women and greater than 14 drinks per week for men; patients with either past or current alcohol abuse at the time of diagnosis were considered positive for lifetime alcohol abuse (11).
Staging was performed using the 2010 AJCC staging criteria based on radiographic and endoscopic data (esophagoscopy, accompanied by biopsy, endoscopic ultrasound, and bronchoscopy in most cases) (12). Radiographic studies usually included a combination of neck/chest CT, PET/CT, and barium swallow studies.
Loco-regional failure was defined as recurrence at the primary tumor site or regional lymph nodes, including cervical and supraclavicular basins. For loco-regional control (LRC) and distant control (DC), patients were censored for death or at last follow-up. Disease-free survival (DFS) was calculated from the end-date of definitive CRT to the date of first failure or death. Overall survival (OS) was calculated from date of initial diagnosis to date of death.
Survival and failure rates were calculated and plotted using the Kaplan-Meier method (GraphPad Prism Version 5.04). Univariate analysis was performed with log-rank tests, using R package (Version R.2.15) (13). Log-rank tests were performed for each outcome metric (LRC, DC, DFS, and OS) with each of the following variables: tobacco abuse, alcohol abuse, age, AJCC/TNM stage, T component (of TNM staging), N component, tumor length, radiation dose, HPV status, and p16 status. All P values were given for 2-tailed tests, with α=0.05 for all tests.
Immunohistochemistry and chromogenic in situ hybridization (ISH) were performed on available, formalin-fixed, paraffin-embedded tissue samples obtained from the pathology archives. Immunohistochemical staining (IHC) for p16 was performed using the Bond III autostainer (Leica). The pre-dilute antibody (clone 9517, MTM Labs) was used with epitope retrieval for 10 minutes in a citrate buffer. Detection was accomplished with the Leica Refine® polymer system and visualization of the immune complex utilized diaminobenzidine as the chromogen. In the ISH assay, the GenPoint Tyramide Signal Amplification Kit (Dako) was used as specified by the manufacturer in conjunction with the HPV 16/18 oligonucleotide DNA probe (Dako). Pathological studies (p16 IHC and HPV 16/18 ISH) were evaluated and graded as positive or negative by a pathologist specializing in gastrointestinal oncology. Equivocal cases were graded based on consensus of two pathologists.
Results
Patient and tumor characteristics
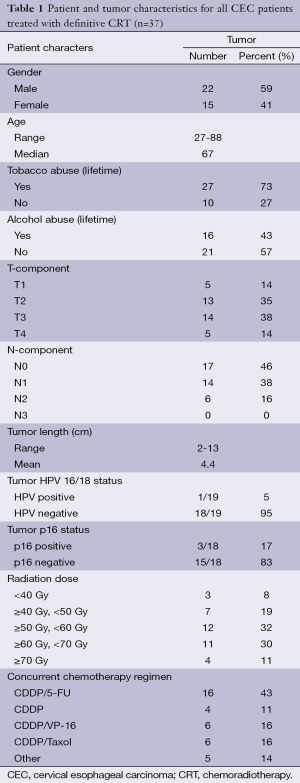
Patient and tumor characteristics are summarized in Table 1. A total of 36 (97.3%) patients had SCC and 1/37 (2.7%) patient had adenocarcinoma.
Full table
Two patients were identified with a primary lesion in the cervical esophagus synchronous with another smaller lesion in the proximal thoracic esophagus. In both cases, clinical, endoscopic, and pathological judgment suggested the smaller lesions represented skip lesions from the primary lesion, and both patients were staged as having T3(m) disease; the distance between the two lesions in both cases was less than 4 cm.
Eight patients were previously treated for other malignancies. Five were treated for oral cavity SCC (two treated with definitive surgery, two with definitive radiotherapy, and one with combined surgery and radiotherapy), and three were treated surgically for breast cancer. For the five cases of prior oral cavity SCC, it was the clinical assessment that the subsequent esophageal lesions represented second primary malignancies, and not recurrences of the prior oral cavity SCC.
Treatment
All patients received definitive CRT; total radiation dose ranged from 14.4 to 71 Gy, with a median radiation dose of 54 Gy (Table 1). All chemotherapy regimens were platinum-based, but varied with regards to the addition of other agents including 5-FU, paclitaxel, and etoposide (VP-16) in the treatment regimen (Table 1). No patients received brachytherapy. Of note, one patient expired during treatment secondary to CVA, after receiving 14.4 Gy and one cycle of CDDP/VP-16; this patient was included in outcomes analysis below unless otherwise specified.
Although no patients underwent definitive surgery, four patients had surgery related to their esophageal cancer. One patient, diagnosed at age 27, initially underwent local excision for suspected leiomyoma, which was then followed by definitive CRT when pathology demonstrated the lesion to be SCC. Three patients underwent surgery following completion of definitive CRT for enlarging neck masses (suspected recurrence), with only one revealing carcinoma.
Outcome measures
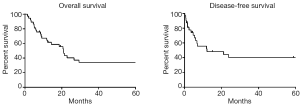
Median follow-up time (for surviving patients) was 129.4 months, and median OS was 20.9 months. Five-year actuarial OS, DFS, LRC, and DC rates were 34.1%, 40.2%, 65.6%, and 65.2%, respectively (Figure 2). Log-rank testing demonstrated that advanced tumor AJCC stage and higher grade significantly predicted increased rates of loco-regional failure (P<0.05). Similarly, advanced tumor stage and advanced patient age at diagnosis significantly predicted poor OS (P<0.05). No other univariate comparisons of patients and tumor characteristics to outcome metrics (see Methods for details) were statistically significant.
Increased radiation dose (≥54 vs. <54 Gy) did not correlate with improved five-year OS (33.6% vs. 41.7%, P=0.982), DFS (32.6% vs. 50.5%, P=0.382), or LRC (62.1% vs. 67.1%, P=0.809). Three patients receiving less than 40 Gy total radiation dose were excluded from this analysis of radiation dose to outcome. All were scheduled to receive at least 40 Gy, but stopped treatment early (one secondary to expiration during treatment, one secondary to rapidly-worsening mental status, and one secondary to severe neutropenia). A total of 40 Gy was established as the minimum radiation dose for this analysis since the two patients receiving palliative-intent CRT for CEC (Figure 1) received 40 Gy or less. Finally, all three curative-intent CRT patients receiving less than 40 Gy expired either during treatment (one patient) or within four months of treatment cessation (two patients).
Two patients developed second malignancies following definitive CRT for CEC. One developed lung adenocarcinoma 10 years following treatment completion. Another developed oral cavity SCC 11 years following treatment completion. Three patients developed chronic esophageal strictures following CRT; two were treated successfully with routine dilatation, and one was treated with PEG placement.
HPV tumor status
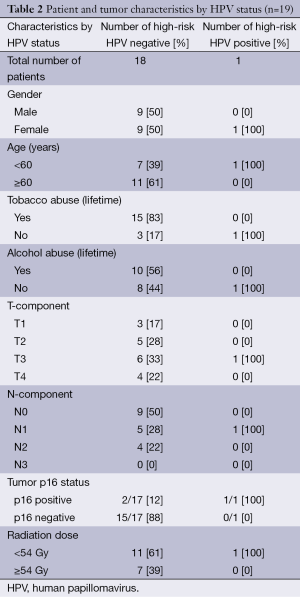
Tumor samples were available for HPV analysis in 19/37 (51.4%) of patients; all samples were from patients with SCC, and characteristics of patients undergoing HPV pathological analysis are shown in Table 2. High-risk HPV (types 16 and 18) ISH revealed positive HPV tumor infection in one patient (1/19, 5.3%). All but one of the tumor samples tested for high-risk HPV were also tested for p16 overexpression (n=18); p16 overexpression is a positive prognostic factor in oropharyngeal cancers and has been correlated with tumor HPV positivity (14,15). Tumor samples from three patients (3/18, 16.7%) were positive for p16, including the sole HPV-positive case. Log-rank tests revealed no statistically significant effect of p16 status on outcome, although p16-overexpressing tumors trended to better outcomes (p16+ vs. p16– median survival: 80.2 vs. 26.7 months, P=0.37).
Full table
Discussion
This study provides long-term outcomes data for CEC patients, and represents the first attempt at characterizing the incidence and prognostic impact of HPV tumor infection in a specific anatomical compartment of the esophagus. Given the involvement of HPV in oropharyngeal SCC, investigators have postulated an ‘anatomical gradient’ may exist in the esophagus, such that rates of HPV tumor infection in esophageal SCC would be higher in the proximal esophagus (8,10). This study represents the first attempt at testing this hypothesis by examining HPV infection rates within SCC lesions of the cervical esophagus. While our data are limited by small sample sizes, the CEC HPV infection rate identified here (5.3%) is consistent with several studies showing <10% HPV SCC infection rates throughout the entire esophagus, particularly in geographical areas of low esophageal SCC HPV prevalence including North America (9). Our data add to the growing body of evidence suggesting that an anatomical gradient of HPV infection within the esophagus may not exist (10).
With previously published data from other sites in the upper gastrointestinal tract, our data also serve to enhance anatomically-precise mapping of tumor HPV involvement. Oropharyngeal SCC tumors appear to have high rates of HPV infection (over 50%) (8), whereas hypopharyngeal and cervical esophageal lesions appear to have lower rates of HPV infection (10.9% and 5.3%, respectively) (16). Thus an anatomical map of HPV SCC infection suggests a steep drop in viral infection rates at sites distal to the oropharynx, including the cervical esophagus.
The patient in our study with positive HPV tumor infection is of interest. This individual, a woman diagnosed with CEC before age 50, has no alcohol or tobacco abuse history. Furthermore, although presenting with AJCC stage IIIA [T3N1M0: tumor invades adventitia (T3), metastases in 1-2 regional lymph nodes (N1), no distant metastasis (M0)] disease, she demonstrated excellent response to definitive CRT (treated to a total radiation dose of 50.4 Gy), and has remained disease-free for 25 months following treatment completion. Of note, all recurrences (loco-regional and distant) in the present study occurred prior to 22 months following treatment completion, with the vast majority (15/16, 93.8%) occurring within 13 months following treatment completion. Other studies also report CEC failures typically occurring within 1 year following treatment completion (6). While interpretation of our data remains limited by small sample sizes, the characteristics of this HPV positive CEC case are unique: a patient with essentially none of the classic risk factors for esophageal SCC and excellent treatment response despite advanced disease.
The conclusions with respect to the impact of HPV tumor infection in CEC are taken with caution, given the limitations in sample size. However, the literature from head and neck (H&N) SCC lesions provides some guidance with respect to our data. The positive prognostic role of HPV in H&N SCC lesions is established, and ongoing randomized clinical trials are now underway to evaluate the role of therapy de-escalation in HPV-positive H&N SCC lesions owing to their increased treatment responsiveness (17). With these data in mind, our study provides initial evidence regarding the incidence and prognostic impact of HPV infection in CEC SCC; however, larger studies are required to more definitively address etiological questions of HPV infection in CEC.
Our study represents one of the largest CEC studies with long-term follow-up to date and adds to a growing literature supporting the use of definitive CRT to treat CEC. Primary surgical treatment of CEC confers 5-year OS rates from 14% to 31% (18-22), while definitive CRT for CEC tends to provide similar if not improved outcomes based on a number of studies, without the associated surgical morbidity. Of note, we chose not to include the 13 patients treated with definitive surgery for CEC at our institution (Figure 1) for any comparative analyses. The basis for this decision was that this group of patients is quite heterogeneous, and might not be directly representative of surgically-treated CEC patients for a number of reasons: (I) treatment strategies among these patients varied including adjuvant chemotherapy, induction CRT, and other therapies; (II) surgical technique for esophagectomy varied between patients; and (III) most of these patients were treated >15 years ago. The 5-year OS rate for these patients was 7.7%, well below the range of 5-year OS rates cited above for surgically-treated CEC patients.
Regarding the potential limitations of definitive CRT versus surgery, Tong et al. (19) suggested that salvage surgery might be required in a substantial fraction of CEC patients treated with definitive CRT. This claim was based on their experience, where 5/21 CRT-treated patients (23.8%) required salvage pharyngo-laryngo-esophagectomy (PLE). Our study, however, points toward a more limited role for surgical salvage in this population. Three patients (8.1%) underwent regional surgery for presumed neck failure, with only one demonstrating local failure; none required PLE with associated permanent tracheostomy. Other late effects of CRT were also fairly limited, with three patients (8.1%) developing chronic esophageal strictures (two requiring regular esophageal dilation and one requiring PEG placement). While the role of salvage PLE for loco-regional failure requires further study, the present series demonstrated outcomes comparable, if not improved, to those achieved by primary PLE, without high rates of late effects such as esophageal stricture.
Another proposed approach for primary CEC treatment is definitive radiotherapy alone, as recently published by Cao et al. (18). In this study, the majority (69.6%) of patients was treated with definitive radiotherapy and the remainder treated with combined CRT. The authors concluded that higher radiation doses (≥66 Gy) provided significantly improved 2-year survival. By contrast, both the present study and a recently-published analysis on definitive CRT for CEC found no significant differences in outcomes based on radiation dose (6). These data are consistent with the RTOG 94-05 study, wherein no improvements in outcomes were identified in esophageal cancer patients treated with 64.8 vs. 50.4 Gy (23). The value of definitive radiotherapy alone remains unproven; it is possible that higher radiation doses in the absence of concurrent chemotherapy result in improved outcomes. However, given that a prior randomized trial demonstrated the superiority of CRT over radiation alone in treatment of esophageal cancer (24), we recommend definitive CRT as the most appropriate treatment option in selected CEC patients.
In summary, our study supports existing evidence for the use of CRT for CEC. Given the rarity of this lesion and the limited patient numbers along with poor overall outcomes, further investigation of novel therapeutic approaches is warranted. While our study provides an initial glimpse into the role of HPV tumor infection in CEC, larger studies are required to determine the etiological role of HPV infection in subsets of CEC cases. Further understanding of the therapeutic implications of CEC HPV tumor status may allow treatment personalization for this uncommon malignancy.
Acknowledgements
The authors thank David N. Howell for assistance in grading equivocal pathological cases, Robert W. Clough for assistance with data collection, and the Department of Radiation Oncology, Duke University Medical Center, for support of this work.
Authors’ contributions: E.B.L., M.P., X.Z., C.G.W., and B.G.C. designed the overall study. E.B.L. and B.G.C. collected and reviewed clinical data. X.Z. performed and graded pathological studies. E.B.L. and Y.W. analyzed data. E.B.L., M.P., X.Z., C.G.W., and B.G.C. wrote the manuscript.
Funding: This work was supported by the Department of Radiation Oncology, Duke University Medical Center.
Disclosure: The authors declare no conflict of interest.
References
- Mendenhall WM, Sombeck MD, Parsons JT, et al. Management of Cervical Esophageal Carcinoma. Semin Radiat Oncol 1994;4:179-91. [PubMed]
- Mendenhall WM, Parsons JT, Vogel SB, et al. Carcinoma of the cervical esophagus treated with radiation therapy. Laryngoscope 1988;98:769-71. [PubMed]
- Stuschke M, Stahl M, Wilke H, et al. Induction chemotherapy followed by concurrent chemotherapy and high-dose radiotherapy for locally advanced squamous cell carcinoma of the cervical oesophagus. Oncology 1999;57:99-105. [PubMed]
- Burmeister BH, Dickie G, Smithers BM, et al. Thirty-four patients with carcinoma of the cervical esophagus treated with chemoradiation therapy. Arch Otolaryngol Head Neck Surg 2000;126:205-8. [PubMed]
- Yamada K, Murakami M, Okamoto Y, et al. Treatment results of radiotherapy for carcinoma of the cervical esophagus. Acta Oncol 2006;45:1120-5. [PubMed]
- Gkika E, Gauler T, Eberhardt W, et al. Long-term results of definitive radiochemotherapy in locally advanced cancers of the cervical esophagus. Dis Esophagus 2014;27:678-84. [PubMed]
- Hancock SL, Glatstein E. Radiation therapy of esophageal cancer. Semin Oncol 1984;11:144-58. [PubMed]
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24-35. [PubMed]
- Liyanage SS, Rahman B, Ridda I, et al. The aetiological role of human papillomavirus in oesophageal squamous cell carcinoma: a meta-analysis. PLoS One 2013;8:e69238. [PubMed]
- Löfdahl HE, Du J, Näsman A, et al. Prevalence of human papillomavirus (HPV) in oesophageal squamous cell carcinoma in relation to anatomical site of the tumour. PLoS One 2012;7:e46538. [PubMed]
- Dawson DA, Grant BF, Li TK. Quantifying the risks associated with exceeding recommended drinking limits. Alcohol Clin Exp Res 2005;29:902-8. [PubMed]
- Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC Cancer Staging Manual: esophagus and esophagogastric junction. Ann Surg Oncol 2010;17:1721-4.
- Dean CB, Nielsen JD. Generalized linear mixed models: a review and some extensions. Lifetime Data Anal 2007;13:497-512. [PubMed]
- Fischer CA, Kampmann M, Zlobec I, et al. p16 expression in oropharyngeal cancer: its impact on staging and prognosis compared with the conventional clinical staging parameters. Ann Oncol 2010;21:1961-6. [PubMed]
- Blitzer GC, Smith MA, Harris SL, et al. Review of the clinical and biologic aspects of human papillomavirus-positive squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2014;88:761-70. [PubMed]
- Joo YH, Lee YS, Cho KJ, et al. Characteristics and prognostic implications of high-risk HPV-associated hypopharyngeal cancers. PLoS One 2013;8:e78718. [PubMed]
- Psyrri A, Sasaki C, Vassilakopoulou M, et al. Future directions in research, treatment and prevention of HPV-related squamous cell carcinoma of the head and neck. Head Neck Pathol 2012;6 Suppl 1:S121-8. [PubMed]
- Cao C, Luo J, Gao L, et al. Definitive radiotherapy for cervical esophageal cancer. Head Neck 2013. [Epub ahead of print]. [PubMed]
- Tong DK, Law S, Kwong DL, et al. Current management of cervical esophageal cancer. World J Surg 2011;35:600-7. [PubMed]
- Kakegawa T, Yamana H, Ando N. Analysis of surgical treatment for carcinoma situated in the cervical esophagus. Surgery 1985;97:150-7. [PubMed]
- Triboulet JP, Mariette C, Chevalier D, et al. Surgical management of carcinoma of the hypopharynx and cervical esophagus: analysis of 209 cases. Arch Surg 2001;136:1164-70. [PubMed]
- Nishimaki T, Kanda T, Nakagawa S, et al. Outcomes and prognostic factors after surgical resection of hypopharyngeal and cervical esophageal carcinomas. Int Surg 2002;87:38-44. [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [PubMed]
- Herskovic A, Al-Sarraf M. Combination of 5-Fluorouracil and Radiation in Esophageal Cancer. Semin Radiat Oncol 1997;7:283-290. [PubMed]


