Phase Ib trial of gemcitabine with yttrium-90 in patients with hepatic metastasis of pancreatobiliary origin
Introduction
The incidence of liver metastasis of pancreaticobiliary origin, with approximate median survival of 6 months, is increasing worldwide (1,2). Majority of these patients have locally advanced or metastatic disease at time of diagnosis, and are not candidates for curative surgery (1,2). Currently, treatment options are limited to systemic therapy (3), though locoregional therapies have been introduced as a new alterative treatment.
Yttrium-90 radioembolization (90Y-RE) is a promising locoregional therapy for treating liver metastasis of pancreaticobiliary origin (4,5). Our prior 90Y-RE studies on patients with unresectable intrahepatic cholangiocarcinoma (ICC) unresponsive to chemotherapy as well as hepatic metastasis from pancreatic cancer (PC) showed acceptable safety profile for 90Y-RE with improved overall survival (5-7).
A combination of locoregional and systemic therapies is a strategy to enhance the effect of radioembolization or chemotherapy alone. 90Y-RE works by inducing radiation injury, and agents which could increase radiosensitivity of tumor cells could be theoretically beneficial (8). Gemcitabine-based chemotherapies are standard of care for patients with pancreaticobiliary origin (9), and can amplify effects of radiotherapy in locally advanced ICC and PC through potent radiosensitizer effect (10,11). Although both therapeutic approaches have been independently studied in hepatic metastasis of pancreatic or biliary origin with some retrospective studies analyzing combination of 90Y-RE with different chemotherapies (12), no prior clinical trial has studied the safety and feasibility of a combination of 90Y-RE and gemcitabine therapy in hepatic metastasis of pancreaticobiliary origin. This clinical trial was designed to investigate safety and feasibility of this combination therapy in this highly vulnerable patient population for the first time.
Methods
Study design and objectives
This was a prospective single-institute IRB approved Health Insurance Portability and Accountability Act-compliant safety/feasibility quasi-trial. Patients with hepatic dominant pancreatobiliary cancers were enrolled for a combination of glass-based 90Y-RE and gemcitabine (clinicaltrials.gov NCT01434459). Investigational Device Exemption (IDE) of the Yttrium-90 TheraSphere® was obtained from the Food and Drug Administration (FDA) prior to the clinical trial. The study was approved by institutional IRB committee, and informed consent was obtained from all subjects prior to the study.
The primary aim was to determine the recommended phase II dose gemcitabine in combination with 90Y-RE in patients with unresectable hepatic metastasis of pancreaticobiliary origin. Secondary objectives included characterizing toxicities associated with combination of gemcitabine and 90Y-RE, evaluating the hepatic progression free survival (HPFS), and determining the post treatment response rate and progression free survival (PFS) of the enrolled patients.
Study population
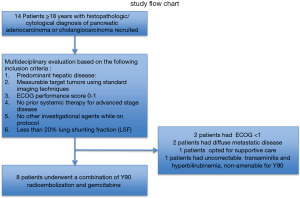
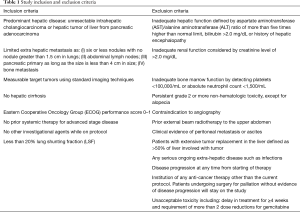
Patients were assessed by a multidisciplinary tumor board, the most optimal treatments were discussed and were found to meet the criteria for the inclusion and exclusion criteria of the study (Table 1).
Full table
Demographics and baseline disease characteristics as well as history of previous therapies were obtained during the patients’ first office visit. Further evaluations were performed at the time of gemcitabine administration, during which data regarding gemcitabine dose (including any modifications or discontinuation), possible concomitant treatments, outcome, and any adverse events were recorded.
Patients 18 years or older who had histopathologic/cytological diagnosis of pancreatic adenocarcinoma or cholangiocarcinoma were screened for the study’s inclusion and exclusion criteria (Table 1). Patients who met the inclusion criteria were enrolled into this study.
Gemcitabine, toxicity and dose modifications
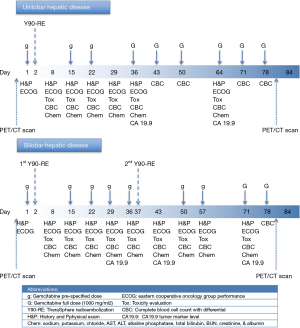
This study was designed as an open label 3+3 phase I with dose escalation of gemcitabine (Gemzar®, Eli Lilly and Company, Indianapolis, IN, USA), which remains a prevailing method for conducting phase I cancer clinical trials. The dose levels for gemcitabine were determined as dose level 1 equals to 400 mg/m2, dose level 2 of 600 mg/m2, dose level 3 of 800 mg/m2, and dose level 4 of 1,000 mg/m2 (Figure 1). This design planned to enroll three patients in cohort at level 1 dose (400 mg/m2) which is considered safe based on animal or prior human toxicological data, and then escalated the dose level at certain time intervals shown on Figure 1 till reach the predetermined goal dose for each group of 3 patients.
Subjects enrolled into this study were carefully monitored during the entire treatment by consistently detailed recording of any adverse events, medical interviews, physical examinations and blood pressure as well as laboratory workup.
Toxicity grading was performed based on the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.3 (13). Toxicities to gemcitabine may include hematologic, gastrointestinal, fever, rash, pulmonary, edema, flu-like symptoms, infection, alopecia, extravasation, allergic reaction, and cardiovascular. For any grade 1 toxicity, treatment was continued at the same dose. Dose limiting toxicity (DLT) was determined as grade 3 non-hematologic toxicity lasting >7 days, grade 4 hematologic toxicity lasting ≥7 days, and any grade 4 non-hematologic toxicity during the first 28 days of therapy. Maximal tolerated dose (MTD) was defined as the highest dose level at which less than two patients develop a DLT in the first 28 days of treatment.
The enrollment scheme was the conventional 3+3 design. Patients were enrolled in cohorts of three. If 0/3 or 1/6 patients treated at a certain dose level have DLT, then three more patients are enrolled at the next dose level. If two patients have DLT on a set dose level, then dose de-escalation was performed and three additional patients are enrolled to trial that dose level to reevaluate safety. Dose escalation continued until either two patients on a specific dose developed dose limiting toxicities or until completion of dose level 4. Once the dose of any of the drugs was reduced it was not increased later on. A maximum of two dose reductions per drug was allowed after which the drug had to be discontinued. In the case of multiple toxicities, dose adjustment was done as per the worst toxicity.
Y90 glass microsphere radioembolization therapy planning
Concurrent with lung shunting fraction (LSF) study, diagnostic angiography of visceral arteries including celiac trunk and superior mesenteric artery was also done to determine the arterial blood supply to the liver and targeted tumor(s). Technically, selective angiography was subsequently performed using a 2.8 French microcatheter by placing the tip of the catheter at the origin of the common hepatic artery. Coil embolization of the right or left gastric branches or gastroduodenal artery was performed, if any contrast flow was noted into the respective artery to avoid non-targeted embolization of the stomach or duodenum. No other artery in this vicinity required coil embolization. Proximity of targeted tumor(s) to the mucosal organs was not considerd as limiting factor. Then, 148 MBq (or 4 mCi) of technetium 99-macroaggregated albumin was injected through the microcatheter, followed by single-photon emission computed tomography (SPECT)/computed tomography (CT) study to evaluated for LSF, as previously detailed in literatures (14).
Patients with an LSF <20% who met the above mentioned inclusion criteria underwent glass-based 90Y-RE (TheraSphere®, BTG, West Conshohocken, PA, USA) therapy after detailed treatment planning according to the manufacturer’s guidelines, including 120-Gy dosimetry of the treated liver lobe (15). For patients with bilobar disease, the targeted lobar dosimetry for each lobe was also 120 Gy. Each lobe was addressed in a separate treatment session, 35 days apart from each other, on day 37th (Figure 1). In an outpatient setting, 90Y-RE was performed according to previously described guidelines (16).
Treatment schedule
Gemcitabine was administered by intravenous infusion over 30 minutes starting at day 1 followed by 90Y-RE to the first lobe on day 2. For patients with bilobar disease, the 90Y-RE treatment of the second lobe performed on day 37. Gemcitabine treatment schedule for patients with unilobar and bilobar disease is shown in Figure 1.
The patients were pre-medicated with a 5HT3 antagonist prior to administration of chemotherapy to prevent nausea and vomiting prophylactically.
Clinical, laboratory and imaging follow-up
In addition to the pretreatment evaluations, all enrolled patients were also followed up by planned office visits, obtaining detailed medical history, performing physical examination, evaluating ECOG performance status at the Interventional Oncology Clinic at certain intervals shown in Figure 1.
Complete blood cell count with differentiation and complete blood biochemistry panel were also ordered to assess the patients for any organ-specific toxicity based on CTCAE (13), in addition to CA 19-9 tumor marker. If any adverse event required hospitalization, the patient was admitted to the interventional radiology unit.
Baseline images were performed within 4 weeks before starting medication, and end examination was performed between day 78 and 84. Patients were imaged on a GE Discovery MV690 PET/CT scanner (GE Healthcare, Wisconsin, USA) after at least 4 hours of fasting. First, an initial CT scan of Abdomen was conducted with administration of intravenous and oral contrast only at 80 to 120 mA and 120 kVp. After this, patients were injected 18F-FDG with an activity ranging from 2.23 to 15.21 MBq/kg. Whole-body images corrected for attenuation (3 min emission, 2 min transmission/bed position) was acquired 60 min after tracer administration and data were transferred to a MIM Vista workstation (MIM Software; Cleveland, Ohio, USA) for analysis.
Imaging analysis
Two radiologists with nuclear medicine fellowship background interpreted images on the basis of pre- and post-treatment PET scans using high-resolution picture and archiving communication system (PACS) workstations. If any discrepancy happened in interpreting the response to treatment, images were reviewed together by both radiologists, and a consensus was reached.
Tumor response was assessed based on the revised Response Evaluation Criteria in Solid Tumors version (RECIST) 1.1 (17). All measurable tumors up to a total of 5 lesions were considered target lesions. All assessed tumors were selected based on the size and suitability for repeat accurate measurements. The longest diameter of all target lesions was summed and reported as the baseline sum LD, which was then used as a reference to assess objective response. The remaining lesions were marked as non-target lesions and followed only as absent or present.
Lesions measured ≥20 mm on multi-slice CT scan or ≥10 mm on spiral CT scan in at least one dimension were considered measurable. All other lesions were considered non-measurable including all lesions with longest diameter of <20 mm on multi-slice CT scan, ascites, and pleural effusions.
Response by F18-FDG-PET scan was carried out based on a composite standardized uptake values (SUV) score obtained for all the lesions in each hepatic lobe. The change in the SUV score was evaluated pre and post 90Y-RE. Positron Emission Tomography (PET) Response Criteria in Solid Tumors (PERCIST) version 1.0 was used for objective evaluation of tumor response (18). Objective criteria were implicated to define response to the treatment as complete response (CR), partial response (PR), stable disease (SD) or progressive disease (PD) (19).
Statistical analysis
SPSS statistical software version 22.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis and data management. The quantitative data was presented as mean ± standard deviation (SD) and range (minimum–maximum), while qualitative data was presented as number (N) and percentage (%). Pearson’s Chi-square test was applied to evaluate the patients’ baseline characteristics and clinical variables and eventually determine predictors of adverse outcomes. PFS was the time interval between the registration date and disease progression or death, while HPFS was calculated as the time interval between the enrollment to the date of disease progression in the treated hepatic lobe. This endpoint is commonly used to evaluate the efficacy of the liver directed therapies (20). Kaplan-Meier estimation was performed to determine the survival from the day of enrollment. Various plausible prognostic factors were investigated using univariate analysis by applying a log-rank test. These variables included the patient’s age, sex, ethnicity, baseline ECOG performance status, tumor burden, any comorbidity, liver function status based on serum albumin level, serum bilirubin level, ascites, portal hypertension, and encephalopathy, as well as LSF. P value <0.05 was considered significant.
Results
Patients and demographic data
Overall, 14 patients with hepatic metastasis with pancreaticobiliary origin were screened for this study from May 2011 to July 2013. Eight patients, three with PC and five with ICC, underwent 90Y-RE. Six patients excluded from the study due to meeting the exclusion criteria. The study flow chart and excluded patients are illustrated in Figure 2.
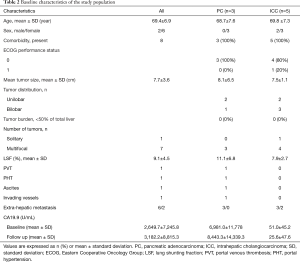
Baseline demographic characteristics of the studied patients are summarized in Table 2. The mean age of the treated patients was 69.4±6.9 years in a predominantly female (75%) and white cohort (100%).
Full table
The mean interval between the diagnosis and initial 90Y-RE was 6.7±6.2 months. All patients received single course of 90Y-RE for each diseased liver lobe. In patients who had bilobar liver disease (50%), the combination treatments for both diseased lobes was defined a single course. The mean Y90 radiation dose delivered to each lobe of the liver was 115.3±11.42 Gy.
Side effects and complications
There was no non-targeted embolization observed based on post-90Y-RE Bremsstrahlung SPECT/CT scan. No 90Y-RE related complications such as gastrointestinal ulceration or pneumonitis was seen on post-90Y-RE Bremsstrahlung SPECT/CT scan. The Table 3 shows the side effects and complications of 90Y-RE and gemcitabine combination therapy. No radiation induced pneumonitis, gastrointestinal ulcer, or renal toxicity was reported. Gemcitabine was escalated up to dose level 3 (800 mg/m2) in 2 patients. Transient fatigue occurred in all patients (100%) and was the most common treatment-associated complication. The second most common complication was nonspecific mild abdominal pain which was reported by 2 patients (25%). These symptoms were resolved without any intervention within the first 3 weeks after therapy.
Full table
Seven patients (87.5%) experienced transient liver toxicity after 90Y-RE and gemcitabine. Four patients (50%) had isolated transaminitis, hyperbilirubinemia or elevated alkaline phosphatase (Table 3). Two patients (25.0%) developed concomitant grade 3 bilirubin toxicity and grade 3 transaminitis, while single patient had grade 2 transaminitis and concurrent grade 2 elevated alkaline phosphatase. No gemcitabine related toxicity is seen with dose level 1 and 2. All hepatic toxicities were observed with dose level 4, except in one patient who developed grade 3 transaminitis and hyperbilirubinemia after dose level 3.
One patient with transient grade 3 hepatobiliary toxicity required hospitalization with 5-day length of stay. Patients who had grade 2 toxicities (n=2) were admitted to the hospital. However, the patients with grade 3 toxicities (n=3) were admitted to the medical intensive care unit (MICU) to ensure close observation and sufficient supportive care. These toxicities were transient and resolved within 30 days of the initial diagnosis. No sequela of hepatobiliary toxicity was reported within 90 days.
Objective tumor response rate and progression
Of the 8 patients, 7 (89.5%) were available to evaluate the tumor response after 3 months, and one patient died at 1.2 month after 90Y-RE.
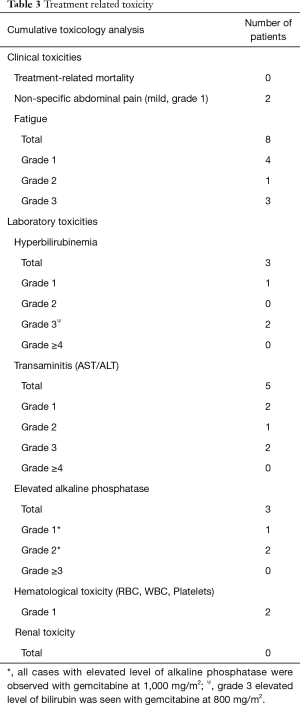
The cumulative HPFS curve for all patients with hepatic metastasis with pancreaticobiliary origin is shown in Figure 3A. The median HPFS for all patients was 8.71 months (1.2–28.4 months). The cumulative HPFS curve for PC and ICC subgroups is shown in Figure 3B. The median HPFS for PC vs. ICC patients were 2.37 vs. 20.70 months (1.2–4.5 vs. 2.7–28.4 months; P<0.001).
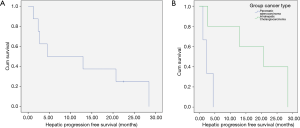
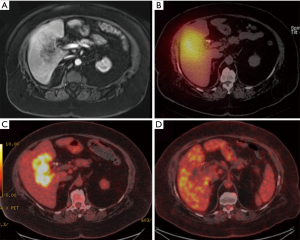
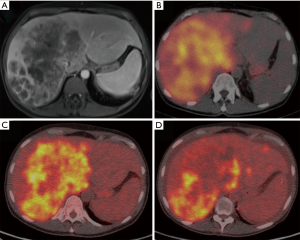
A case of complete response to gemcitabine and 90Y-RE is shown in Figure 4, while Figure 5 demonstrate a case of partial response to gemcitabine and 90Y-RE based on PET/CT scan obtained 3 months after radioembolization. The summary of objective response at 3 months for all patients as well as based on of PC and ICC subtypes are demonstrated in Table 4. The treatment response rate (complete and partial responses) was 62.5% (5/8) at 3 months and disease control rate was 50% (4/8) after 6 months following 90Y-RE treatment, with only one complete response. Overall, the ICC subtype had better objective response rate compared to the PC subtype (P=0.004).
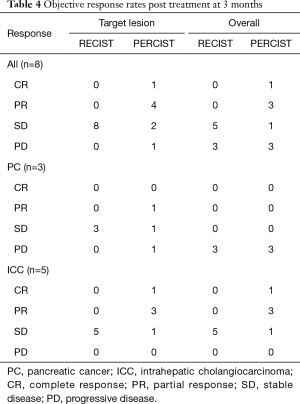
Full table
The cumulative PFS curve for all patients with hepatic metastasis with pancreaticobiliary origin is shown in Figure 6A. The median PFS for all patients was 6.9 (1.2–22.4) months. The cumulative PFS curve for PC and ICC subgroups is shown in Figure 6B. The median HPFS for PC vs. ICC patients were 4.4 (1.2–4.9) vs. 16.3 (2.7–22.5) months (P<0.001).
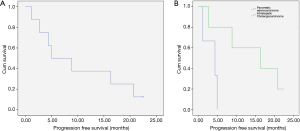
Following 90Y-RE and gemcitabine therapy, ECOG performance was increased in 5 patients; one patient’s ECOG increased from 2 to 3 during first month, in another patient ECOG increased from 0 to 1 during second month of follow-up, and in three patients, ECOG improved from 0 to 2 during third month of follow-up.
Discussion
Our study demonstrated that a combination of 90Y-RE and gemcitabine up to 600 mg/m2 is safe for patients with unresectable hepatic metastasis with pancreaticobiliary origin. No treatment-related mortality is observed during the first 30 days post treatment.
The rationales for combining of 90Y-RE and gemcitabine in hepatic metastasis with pancreaticobiliary origin include gemcitabine as a potent radiosensitizer to enhance the sensitivity of tumor cells to local radiation therapy. 90Y-RE, as a locoregional radiation therapy, independently results in to DNA breaks and catastrophic cellular injury which interrupts maintenance of the genetic integrity resulting in cell death by a variety of mechanisms including mitotic catastrophe, apoptosis, necrosis, and autophagy (21). Combination with Gemcitabine (deoxycytidine) could independently interfere with DNA synthesis, termination of DNA elongation and decreased fidelity of DNA replication (22). However, gemcitabine not only destroy tumor cells by cytotoxic action but also additionally as a potent radiosensitizer of human tumor cells enhances the effects of any radiotherapy. Multiple mechanisms are shown in preclinical studies the role of gemcitabine enhancing radiosensitivity including gemcitabine-induced dATP depletion, withholding cells in the S phase of the cell cycle causing apoptosis, significant increase in DNA double strand breaks and residual DNA damage, or increased enzymatic activity of Dck (22). Transnational studies shown that a combination of gemcitabine and radiation therapies abrogates G2 arrest, impairs DNA damage repair, enhances tumor cell apoptosis by activation of p73/GADD45, down regulates BRCA1-associated genome surveillance complex (BASC) interrupting sensation of abnormal DNA structure and DNA damage repair, causing genetic instability and leading to suppression of cell proliferation in response to radiation therapies (23).
The recommended dosage of gemcitabine is 1,000 mg/m2 intravenously over 30 minutes once weekly for the first 7 weeks, with one week rest, and then once weekly for 3 weeks of each 28-day cycle. However, adding gemcitabine into radiotherapies can induce radio-sensitization in tumor cells at the concentrations 1,000 times lower than the regular plasma levels. The radio-sensitizing effect of these gemcitabine concentrations is dose and schedule dependent, and even could be induced by a short 2-hour exposure to high dose or a long 24-hour exposure to low dose of gemcitabine (22,24,25). Thereby, we hypothesized enhancing patients’ outcomes by combing two therapies in synergistic ways. This study investigated potential risk of the combination therapies.
Post 90Y-RE complications and side effects are categorized into post-radioembolization syndrome, gastrointestinal ulceration, hepatic dysfunction, biliary squeal, portal hypertension, radiation pneumonitis, vascular injury, lymphopenia and a miscellaneous category (26). Hepatobiliary and bone marrow side effects of gemcitabine have been described, and addressed by many previously published studies (6,27). Although 62.5% of patients developed transient toxicities in our study, toxicities observed were mile and transient, managed with medical therapy or with observation, consistent with prior reports (28). The safety was achieved delivering high dose of lobar liver radiation with a mean dose of 115.3 Gy, while external beam radiotherapies deliver a radiation dose of 20 to 60 Gy within 1 cm from the source (29). None had 90Y-RE related complication or synergistically added side effect in combination with gemcitabine.
The ECOG performance status, tumor type, focality of hepatic disease, and tumor burden has been reported to influence overall survival (27,28). Specially, higher ECOG performance score is associated with a better survival rate (28). Our study demonstrated improved ECOG performance after 90Y-RE. ECOG performance status was improved in 62.5% of patients, and same percentage of patients responded to the combination therapy, demonstrating a median OS of 13.8 months.
Previous studies on efficacy of 90Y-RE in unresectable hepatic metastasis with PC or ICC origin demonstrated disease control rate between 57% and 100% after 3 months (6,27,28,30-32). 90Y-RE after cisplatin/gemcitabine chemotherapy retrospectively reported a disease control rate of 81.8% at 3 months in patients with unresectable ICC (28). Our study demonstrated overall disease control rate of 100% in ICC subtype and 67% in targeted hepatic lesion of PC origin after 3 months.
With preliminary reports suggesting benefit for 90Y-RE in hepatic metastasis with pancreatobiliary origin, this quasi trial was designed to assess safety and feasibility of a combination of 90Y-RE and gemcitabine combination therapy. Majority of the prior studies were limited due to the retrospective design. With the safety and efficacy signal particularly in ICC of the current prospective trial warrants randomized efficacy clinical trials to investigate the efficacy of 90Y-RE and gemcitabine combination therapy on unresectable hepatic metastasis of pancreaticobiliary origin. However, this was a small clinical randomized trial with only 8 patients. In addition, this study had to be terminated before completing enrollment of required number of the patients, due to lack of enough chemotherapy naive patients. Furthermore, the dose of gemcitabine could only be escalated up to level 3 (800 mg/m2). Therefore, a multicenter study should be considered in follow-up phases to ensure enrollment of enough therapy native patients with hepatic metastasis of pancreaticobiliary tumors.
In conclusion, a combination of Y90 radioembolization and 600 mg/m2 gemcitabine concomitant therapy is a safe and feasible treatment option which potentiates palliative control of unresectable hepatic metastasis with pancreaticobiliary origin without significant additive toxicities. This approach is a viable treatment option and warrants further trials for evaluation of efficacy.
Acknowledgments
Funding: This study was supported by BTG (West Conshohocken, PA). HSK is supported by the United States Department of Defense (CA160741).
Footnote
Conflicts of Interest: HS Kim served on Advisory boards for Boston Scientific and SIRTex. The other authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Institutional Review Board at the Emory University. All patients were consented in order to be rolled into the study and use their secured personal data and clinical information for the purposes of the study. We did not enrolled patients younger than 18-year-old. The study outcomes did not affect the future management of the patients.
References
- Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: a predictor for patient outcome. Cancer 2012;118:3182-90. [Crossref] [PubMed]
- Hamrick RE Jr, Liner FJ, Hastings PR, et al. Primary carcinoma of the gallbladder. Ann Surg 1982;195:270-3. [Crossref] [PubMed]
- Halfdanarson TR, Haraldsdottir S, Borad MJ. Advances in systemic therapy for advanced pancreatobiliary malignancies. F1000Res 2013;2:105. [Crossref]
- Michl M, Haug AR, Jakobs TF, et al. Radioembolization with Yttrium-90 microspheres (SIRT) in pancreatic cancer patients with liver metastases: efficacy, safety and prognostic factors. Oncology 2014;86:24-32. [Crossref] [PubMed]
- Ludwig JM, Ambinder EM, Ghodadra A, et al. Lung Shunt Fraction prior to Yttrium-90 Radioembolization Predicts Survival in Patients with Neuroendocrine Liver Metastases: Single-Center Prospective Analysis. Cardiovasc Intervent Radiol 2016;39:1007-14. [Crossref] [PubMed]
- Rafi S, Piduru SM, El-Rayes B, et al. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: survival, efficacy, and safety study. Cardiovasc Intervent Radiol 2013;36:440-8. [Crossref] [PubMed]
- Nezami N, Kokabi N, Camacho JC, et al. Y90 radioembolization dosimetry using a simple semi-quantitative method in intrahepatic cholangiocarcinoma: Glass versus resin microspheres. Nucl Med Biol 2018;59:22-8. [Crossref] [PubMed]
- Andren-Sandberg A. Pancreatic cancer: chemotherapy and radiotherapy. N Am J Med Sci 2011;3:1-12. [Crossref] [PubMed]
- Park JO, Oh DY, Hsu C, et al. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res Treat 2015;47:343-61. [Crossref] [PubMed]
- Shewach DS, Lawrence TS. Antimetabolite radiosensitizers. J Clin Oncol 2007;25:4043-50. [Crossref] [PubMed]
- Small W Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol 2008;26:942-7. [Crossref] [PubMed]
- Edeline J, Du FL, Rayar M, et al. Glass Microspheres 90Y Selective Internal Radiation Therapy and Chemotherapy as First-Line Treatment of Intrahepatic Cholangiocarcinoma. Clin Nucl Med 2015;40:851-5. [Crossref] [PubMed]
- Services UDoHaH. Common Terminology Criteria for Adverse Events (CTCAE) version 4.03. 2009. Available online: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed February 11, 2014.
- Kokabi N, Galt JR, Xing M, et al. A simple method for estimating dose delivered to hepatocellular carcinoma after yttrium-90 glass-based radioembolization therapy: preliminary results of a proof of concept study. J Vasc Interv Radiol 2014;25:277-87. [Crossref] [PubMed]
- Salem R, Thurston KG, Carr BI, et al. Yttrium-90 microspheres: radiation therapy for unresectable liver cancer. J Vasc Interv Radiol 2002;13:S223-9. [Crossref] [PubMed]
- Salem R, Lewandowski RJ, Gates VL, et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol 2011;22:265-78. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- O JH. Lodge MA, Wahl RL. Practical PERCIST: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016;280:576-84. [Crossref] [PubMed]
- Camacho JC, Kokabi N, Xing M, et al. PET response criteria for solid tumors predict survival at three months after intra-arterial resin-based 90Yttrium radioembolization therapy for unresectable intrahepatic cholangiocarcinoma. Clin Nucl Med 2014;39:944-50. [Crossref] [PubMed]
- Gill S, Berry S, Biagi J, et al. Progression-free survival as a primary endpoint in clinical trials of metastatic colorectal cancer. Curr Oncol 2011;18 Suppl 2:S5-10. [Crossref] [PubMed]
- Chaudhry MA. Biomarkers for human radiation exposure. J Biomed Sci 2008;15:557-63. [Crossref] [PubMed]
- Pauwels B, Korst AE, Lardon F, et al. Combined modality therapy of gemcitabine and radiation. Oncologist 2005;10:34-51. [Crossref] [PubMed]
- Yong KJ, Milenic DE, Baidoo KE, et al. Cell Killing Mechanisms and Impact on Gene Expression by Gemcitabine and 212Pb-Trastuzumab Treatment in a Disseminated i.p. Tumor Model. PLoS One 2016;11:e0159904. [Crossref] [PubMed]
- Shewach DS, Lawrence TS. Gemcitabine and radiosensitization in human tumor cells. Invest New Drugs 1996;14:257-63. [Crossref] [PubMed]
- Lawrence TS, Eisbruch A, Shewach DS. Gemcitabine-mediated radiosensitization. Semin Oncol 1997;24:S7-24-S7-28.
- Riaz A, Lewandowski RJ, Kulik LM, et al. Complications following radioembolization with yttrium-90 microspheres: a comprehensive literature review. J Vasc Interv Radiol 2009;20:1121-30. [Crossref] [PubMed]
- Mouli S, Memon K, Baker T, et al. Yttrium-90 radioembolization for intrahepatic cholangiocarcinoma: safety, response, and survival analysis. J Vasc Interv Radiol 2013;24:1227-34. [Crossref] [PubMed]
- Jia Z, Paz-Fumagalli R, Frey G, et al. Resin-based Yttrium-90 microspheres for unresectable and failed first-line chemotherapy intrahepatic cholangiocarcinoma: preliminary results. J Cancer Res Clin Oncol 2017;143:481-9. [Crossref] [PubMed]
- Nakeeb A, Pitt HA. Radiation therapy, chemotherapy and chemoradiation in hilar cholangiocarcinoma. HPB (Oxford) 2005;7:278-82. [Crossref] [PubMed]
- Cao C, Yan TD, Morris DL, et al. Radioembolization with yttrium-90 microspheres for pancreatic cancer liver metastases: results from a pilot study. Tumori 2010;96:955-8. [Crossref] [PubMed]
- Kalinowski M, Dressler M, Konig A, et al. Selective internal radiotherapy with Yttrium-90 microspheres for hepatic metastatic neuroendocrine tumors: a prospective single center study. Digestion 2009;79:137-42. [Crossref] [PubMed]
- Savic LJ, Chapiro J, Geschwind JH. Intra-arterial embolotherapy for intrahepatic cholangiocarcinoma: update and future prospects. Hepatobiliary Surg Nutr 2017;6:7-21. [Crossref] [PubMed]