
Synchronous resection of colorectal primary and hepatic metastasis
Introduction
Approximately 23% to 51% of the 157,000 new colorectal cancer patients will present with synchronous colorectal cancer and liver metastasis (1). Surgical resection of all tumor sites is the only treatment that offers prolonged survival (2-4). However, optimal management of patients with synchronous colorectal hepatic metastasis is complex and must consider multiple factors, including the presence of symptoms, location of primary tumor and liver metastases, extent of tumor (both primary and metastatic), patient performance status, and underlying comorbidities.
When faced with a patient with an asymptomatic primary colorectal cancer, isolated hepatic metastases, and reasonable performance status, a primary consideration when formulating a possible surgical treatment plan involves assessment of resectability of the hepatic metastases. This select group of patients with asymptomatic primary tumors and isolated liver-only metastases can be classified into three groups: (I) diffuse, bilobar, unresectable liver metastases, (II) marginally resectable liver metastases and (III) clearly resectable hepatic metastases. In the first group, most oncologists favor palliative systemic chemotherapy as the primary treatment modality and would reserve surgical management for complications of the primary (e.g. bleeding, obstruction, perforation) or cases where the hepatic metastases may be rendered resectable. In the latter two groups, the following treatment strategies have been employed: (I) resection of the primary followed by systemic chemotherapy followed by liver resection ± additional systemic chemotherapy (Staged approach), (II) systemic chemotherapy followed by simultaneous resection of the primary and hepatic metastases (Synchronous approach) and (III) systemic chemotherapy followed by resection of hepatic metastases followed by resection of the primary (so-called “Reverse Strategy”) (5).
The potential risks and benefits of synchronous compared to staged resections are summarized in Table 1. Advocates of a staged approach endorse this strategy due to concerns about increased morbidity and mortality associated with simultaneous resection of the colorectal primary and hepatic metastases. Concerns about the potential safety and technical feasibility of rectal resections and major hepatic resections have been raised as concerns regarding simultaneous resections (6). In addition, some surgeons and oncologists have pointed to complications associated with the unresected primary tumor as another reason for not adopting synchronous resections (7,8). In contrast, proponents of synchronous resections point to the morbidity associated with multiple procedures as a major advantage of a simultaneous resection approach. From an oncologic standpoint, synchronous resection following neoadjuvant chemotherapy provides insight into the patient’s tumor biology and prevents a delay in administering systemic therapy which may occur due to complications following resection of the colorectal primary.
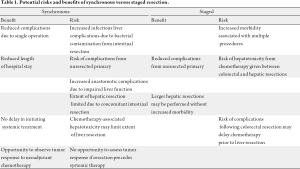
Full table
The current discussion will review the existing literature on staged versus synchronous resection of colorectal cancer and isolated hepatic metastases. Two key issues will be considered: the safety of each resection strategy and oncologic outcomes followed a synchronous versus staged resection. Lastly, we will examine the emerging data available regarding a minimally invasive approach to synchronous colorectal disease with hepatic metastases.
Safety of simultaneous versus staged resections
The first question to be addressed when considering a synchronous versus staged resection for colorectal tumors with hepatic metastases is the safety of each approach. A study by Vogt et al. was among the first to examine the safety of synchronous resection for colorectal cancer (9). The authors compared operative mortality between 19 patients who underwent a synchronous resection to 17 patients who had a staged resection (median 2 months between resections). There were no perioperative deaths in either group. One patient in the synchronous group developed a bile leak (overall complication rate 5%) while three patients in the staged group developed complications (overall complication rate 17.6%). The authors concluded that synchronous resection was associated with a low rate of complication and no operative mortality provided that colorectal resections are not combined with extended liver resections. Five years following the study by Vogt et al. (9), Nordlinger and colleagues (6) compared operative outcomes between 115 patients who underwent a synchronous resection to 893 patients who underwent a staged resection. The operative mortality among the synchronous resections was 7% compared to 2% in the staged resection group. Perioperative morbidity for simultaneous versus staged resections were not reported. Given the increased mortality associated with colorectal resections combined with major liver resections, the authors subsequently adopted a policy of performing synchronous resections only if they can be done with a minor liver resection and through the same abdominal incision. Conversely, when a major liver resection is required to resect synchronous metastases from a rectal carcinoma, for example, they perform the rectal excision first and the liver resection 2 or 3 months later if the liver tumors have remained stable.
Advances in anesthesia and critical care, improved understanding of hepatic anatomy, and better preoperative radiological imaging have led to significant advancements in hepatobiliary surgery in general, and the management of patients with colorectal hepatic metastases specifically. Mortality following major liver resections in current series ranges from 0-5.8% (10-12). Morbidity from these same reports ranges from 22-48% (10-12). As a result, perioperative outcomes following simultaneous resections may also be expected to improve compared to those of the earlier series reported above. Martin and colleagues from Memorial Sloan-Kettering Cancer Center published their experience comparing 134 simultaneous versus 106 staged resections for metastatic colorectal cancer (13). Perioperative mortality was similar in both groups. Using their standard classification scale for complications, they reported a significantly lower complication rate of 48% among simultaneous resection patients compared to a 68% rate among staged resection patients. Importantly, they included the complications sustained during both hospitalizations in the staged resection group. They noted that the difference in the overall complication rate between the simultaneous and the staged group occurred primarily from the need for a second laparotomy in the staged group. An examination of hepatectomy versus colectomy-related complications revealed no difference in procedure-specific complications between the two groups. In 2004, Tanaka et al. (14) published their series of 39 simultaneous and 37 staged resection patients. The perioperative mortality rate was zero in both groups. The morbidity rate was 28% in the simultaneous group compared to 16% in the staged group. The authors noted that although the rate of hepatectomy-related complications (e.g. hyperbilirubinemia, biliary fistula) were slightly higher in the simultaneous resection group the compared to the staged resection group, the results were comparable to those seen in their conventional colorectal hepatic metastasectomy patients. Three years following the report by Tanaka et al. (14), Reddy et al. (15) published a retrospective study of simultaneous or staged colorectal and hepatic resections at three hepatobiliary centers. One hundred and thirty five patients underwent simultaneous and 475 patients underwent staged resection. Mortality and severe morbidity were similar after simultaneous colorectal resection and minor hepatectomy compared with isolated minor hepatectomy. However, increased mortality and severe morbidity was seen following simultaneous colorectal resection and major hepatectomy. Based upon these findings, the authors recommended caution when considering simultaneous colorectal and major hepatic resection but felt simultaneous colorectal and minor hepatic resections were safe and could be recommended for most patients. A smaller study of synchronous versus staged resections for colorectal cancer with hepatic metastases was published by Capussotti in 2007 (16). A major advantage of this study over those described above, however, is that only patients with major liver resections were included. The authors reported their experience in 31 patients who underwent synchronous resection to 48 patients who underwent staged resection. Perioperative mortality occurred in 3.2% of synchronous resection patients and in none of the staged resection patients. Perioperative morbidity occurred in 33% of synchronous resection patients compared to 56% of staged resection patients. Based upon their findings, Capussotti et al. (16) concluded that major hepatectomies can be safely performed at the same time as colorectal surgery in selected patients with synchronous metastases. Furthermore, they did not feel that rectal cancer requiring an anterior resection was a contraindication to synchronous major hepatectomy since 9/31 (29%) of the patients in their synchronous resection group underwent a rectal resection. Thelen et al. (17) sought to clarify the safety of simultaneous liver resections compared to staged hepatectomies and identify criteria of patient selection for simultaneous liver resection. They compared the perioperative outcomes between 40 patients who underwent simultaneous resection to 179 patients who underwent staged resections. The 90-day mortality rate was 10% in the synchronous group compared to 1.1% in the staged group. Morbidity was similar between the two groups: 18% in the simultaneous resection group versus 25% in the staged group. When independent predictors of postoperative mortality were analyzed, only extent liver resection was found to be a significant influence on mortality after simultaneous liver resections. In contrast, none of the demographic or clinical factors investigated had a significant influence on postoperative mortality in the staged resection group.
Martin et al. (18) recently published their experience comparing 70 simultaneous resections of colon primary and liver metastases to 160 patients who underwent staged operations. In contrast to some of the earlier series cited above, the frequency of major liver resections (≥3 Couinaud segments) was similar in the two groups at 33%. The type of primary resection was also similar in the two groups. The postoperative mortality was rate was 2% in both groups. Complication rates were similar in the staged and simultaneous groups: 56% in the simultaneous groups versus 55% in the staged group. The authors concluded that simultaneous resections are safe and acceptable and result in shorter overall length of hospital stay.
In contrast to the above retrospective studies which compared outcomes following synchronous and staged resections for colorectal cancer and hepatic metastases, Moug et al. (19) performed a small case-matched comparison of 32 patients who underwent simultaneous versus staged resections. The patients were matched for age, gender, American Society of Anesthesiologists grade, type of hepatic and colon resection. Major hepatic resections performed were 22% of patients in both groups. There were no postoperative deaths in either group. No significant differences in postoperative morbidity were found between the two groups: the overall morbidity in the synchronous group was 34% compared to 59% in the staged group. The investigators concluded that synchronous resections can be safely performed and noted the absence of any colonic anastomotic leaks, even considering that slightly over one third of the patients underwent a rectal resection with anastomosis. A limitation of this study, however, is the small percentage of patients who underwent a major hepatectomy (resection of ≥3 segments).
A variation on the classic staged approach (colon then liver) has recently been proposed by Brouquet et al. and the group from M.D. Anderson Cancer Center (5). In their “Reverse Strategy” preoperative chemotherapy is followed by resection of the hepatic metastases and then by resection of the colorectal primary at a second operation. The rationale for this approach is based upon the following observations: complications related to the primary colorectal tumor are rare and treatment of metastatic disease is not delayed by local therapy for the primary tumor or complications associated with treatment of the primary tumor. In their study, they examined the perioperative outcomes between 72 patients who underwent a classic staged approach to 43 patients who had a synchronous resection of their primary and metastatic lesions to 27 patients who were treated according to the “Reverse Strategy”. Postoperative mortality rates in the simultaneous, classic, and reverse strategies were 5%, 3%, and 0%, respectively. Postoperative cumulative morbidity rates for the three groups were similar at 47%, 51%, and 31%, respectively. Based upon their findings, the authors concluded that the “Reverse Strategy” can be considered as an alternative option in patients with advanced hepatic metastases and an asymptomatic primary.
In summary, the literature to date supports the safety of a synchronous approach to the resection of colorectal cancer and hepatic metastases (Table 2). Perioperative mortality in most series is ≤5% for either simultaneous or a staged approach. In contrast to the consistently low mortality associated with either a synchronous or staged colorectal and hepatic resection, morbidity rates following these approaches are more variable. One theme does emerge from the available literature, however; morbidity rates are generally increased when colorectal resections are combined with major hepatectomy defined as resection of ≥3 segments. Despite the technical and postoperative improvements associated with hepatic resections over the past decade, most authors recommend caution when considering combining major hepatectomy with colorectal resections. Another risk for increased morbidity among synchronous resection patients is the location of the colorectal primary - specifically the potential for increased morbidity associated with combining rectal and hepatic resections. There appears to be a general trend away from combining rectal resection with hepatic resection although at least one small case-matched study (19) which controlled for this variable failed to show an increase in postoperative morbidity when rectal resections were combined with mostly minor hepatectomy.
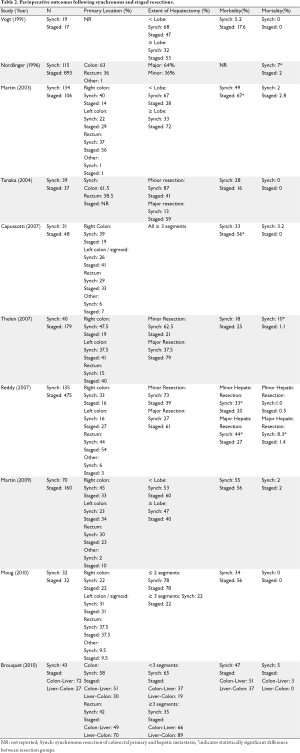
Full table
Oncologic outcomes following simultaneous versus staged resections
Having established the safety of synchronous resection of colorectal and hepatic metastases in select patients, the next key consideration is oncologic outcomes. Do patients who undergo synchronous resections have equivalent (or improved) oncologic outcomes compared to patients who undergo staged resections? In the following section, we will consider overall and disease-free survival rates following simultaneous and staged resections for synchronous metastatic colorectal cancer.
Prior to examining the outcomes following these two resection approaches, it is instructive to review the oncologic outcomes among Stage IV colorectal cancer patients with isolated hepatic metastases treated by standard chemotherapy. A study by Emmanouilides et al. (20) examined outcomes following upfront therapy with bevacizumab, oxaliplatin, leucovorin, and 5-Fluorouracil in chemotherapy-naïve patients with metastatic colorectal cancer. Approximately two-thirds of the patients in their study had liver only as their site of metastasis. A complete response rate of 15% was found following this regimen while partial response was seen in 53%. Time to tumor progression was 11 months. One, two, and three-year survival probabilities were 80%, 64%, and 58%, respectively. Median overall survival had not yet been reached after a median follow-up of 20 months. Despite the significant improvement in time to progression and overall survival associated with modern chemotherapy regimens for metastatic colorectal cancer, the superiority of complete resection, when possible, has been clearly established. In a study of 151 patients with synchronous colon cancer and isolated hepatic metastases, Fahy et al. (4) reported a 5-year disease-specific survival of 54% among resected patients. In contrast, the median survival amongst patients who were not able to undergo hepatic resection was 27 months. This proven superiority of complete surgical resection of colorectal cancer and hepatic metastases over best systemic therapy notwithstanding, in order to evaluate the risks and benefits of a simultaneous versus staged resection, the inherent morbidity and mortality of resectional therapy must compare favorably with best current systemic therapy.
The early study comparing synchronous (N=19) versus staged (N=17) resection of colorectal hepatic metastases by Vogt et al. (9) previously discussed reported an overall median survival in all 36 patients of 28 months. The median overall survival in the synchronous resection group was 18 months with a median disease-free interval of 7 months. Among patients undergoing staged liver resection, median survival was 31 months and disease-free interval was 19 months. Despite this trend toward improved oncologic outcomes following staged resections, the authors concluded that their data do not show an effect of surgical approach on survival. Specifically, an improvement in survival was not seen among simultaneous resection patients.
The advances in surgical technique and perioperative assessment associated with liver resection over the past decade previously discussed have been paralleled by improved systemic therapies for advanced colorectal cancer. Therefore, improved oncologic outcomes may be expected with more current studies since the early report by Vogt et al. (9). In 2004, Tanaka et al. (14) reported their experience with 39 patients who underwent a synchronous colorectal and hepatic resection to 37 patients who underwent staged resections. The overall cumulative 5-year survival rates were similar between the two resection groups at 86% for the simultaneous resection group and 83% for the staged resection group. Disease-free survival was also equivalent between the groups with 5-year rates of 64% and 51% for simultaneous and staged resection groups, respectively. Thelen (17) compared oncologic outcomes between 40 patients who underwent a synchronous resection for colorectal metastases to 179 patients whose disease was resected in a staged fashion. Similar to the findings of Vogt et al. (9) and Tanaka et al. (14), no difference in overall survival was found between the two groups. A multivariate analysis performed to determine predictors of overall mortality identified nodal status of the primary, number of metastases and completeness of hepatic resection (R0 versus R1/R2) as the only independent predictors of mortality. After a mean follow-up of 70 months for all patients, 135/219 patients developed recurrent disease. Recurrent liver-only disease developed in 49 patients, and 12 patients developed both intra- and extrahepatic recurrence. The site(s) of recurrence did not differ between the resection groups. Given the impact of the entire extent of disease burden on oncologic outcomes, the case-matched study by Moug et al. (19) of 32 patients who underwent simultaneous versus staged resections is particularly informative regarding oncologic outcomes following these two resection approaches. As noted above, the patients in this study were matched for age, gender, American Society of Anesthesiologists grade, type of hepatic and colon resection. The overall median survival in the synchronous resection group was 39 months and compared favorably with the median survival of 42 months observed in the staged resection group. Similarly, the median time to cancer recurrence in the synchronous resection group was 10 months, similar to the 14 month disease-free survival seen among the staged resection patients. Although the small sample size is a limitation of this study, these findings provide some provisional evidence that timing of resection does not appear to impact oncologic outcomes adversely.
As discussed previously, the group from M.D. Anderson Cancer Center has recently published their experience with the “Reverse Strategy” toward staged resection of synchronous colorectal hepatic metastases (5). In their study, they reported an overall median survival for the entire population who underwent complete resection of all disease of 64 months. Median survival rates were 95 months in the simultaneous resection group, 55 months in the classic resection group, and 50 months in the “Reverse Strategy” resection group. Overall, 65% of all patients developed recurrent disease: 53% in the combined resection group, 71% in the classic resection group, and 70% in the “Reverse Strategy” group. These recurrence rates were not significantly different. Additionally, median disease-free survivals were the same in the three groups. The authors noted that the outcomes for patients treated with the “Reverse Strategy” who had more extensive disease were similar to outcomes of patients treated with the classic or simultaneous resection groups who had a smaller overall disease burden.
In summary, oncologic outcomes are superior following complete resection of all disease when compared to best available systemic therapies. Additionally, the result of the studies reviewed above indicate that oncologic outcomes, including both overall and disease-free survival, are not different following a synchronous compared to a staged approach to colorectal cancer metastatic to the liver (Table 3). A major limitation to the majority of available studies, however, is the variable extent of disease present among patients subjected to a simultaneous compared to a staged resection approach. Only the study by Moug et al. (19) attempted to address this issue. Based upon their limited case-matched study, the oncologic equivalence seen among the larger studies available appears to be sustained. The “Reverse Strategy” approach (5) is interesting insofar as it provides an approach which allows for extensive hepatectomies to be performed safely in a select group of patients with asymptomatic primary colorectal tumors. The authors have found that this approach helps increase resectability in patients not initially considered candidates for resection and avoids the delay off chemotherapy following initial colorectal resection which may allow for hepatic progression. It is noteworthy that the authors routinely give chemotherapy to all patients with synchronous resection colorectal liver metastases as this is not the routine practice amongst some surgeons who advocate a simultaneous resection for resectable colorectal hepatic metastases.
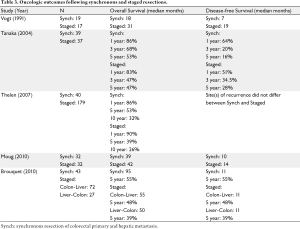
Full table
Role of minimally invasive approaches to synchronous colorectal cancer with hepatic metastases
The safety and efficacy of minimally invasive approaches to colorectal disease, including cancer, was established following the report of the Clinical Outcomes of Surgical Therapy (COST) trial (21) which showed equivalent recurrence and overall survival rates between patients who underwent laparoscopically-assisted compared to open resection for colon cancer. An increase in minimally invasive hepatic resections has paralleled and followed the increased use of minimally invasive approaches to colorectal malignancies. A recent report by Nguyen et al. (22) retrospectively reviewed all cases of minimally invasive hepatectomy for colorectal liver metastases performed in the United States and Europe between 2/2000 - 9/2008. A total of 109 cases were included in the review. Synchronous hepatic lesions were present in 11%. The median interval between resection of the colorectal primary and hepatic resection was 12 months among metachronous patients. Minor hepatectomies (≤3 segments) were performed in 61.5% of patients. The overall complication rate was 12% with no perioperative deaths. Negative margin resections were achieved in 94%. Actuarial overall survival was 88% at one year, 69% at three years, and 50% at 5 years. Disease-free survival for 1-, 3- and 5-years were 65%, 43%, and 43%, respectively. Based upon their review, Nguyen et al. (22) concluded that minimally invasive liver resections for colorectal metastases were feasible and could be performed safely with acceptable safety and oncologic outcomes. Currently, only small single institution series of minimally invasive surgical approaches to synchronous colorectal cancer and hepatic metastases have been published. Kim et al. (23) reported on their initial experience with 10 patients with colorectal cancer and synchronous liver metastases in order to assess the feasibility of a minimally invasive approach to synchronous disease. The primary tumors were resected via anterior or low anterior resection in eight patients, right hemicolectomy in one patient, and subtotal colectomy in one patient. Major hepatectomies were performed in six patients. There were no perioperative deaths. One patient developed postoperative bleeding requiring open re-exploration. The authors concluded that a synchronous minimally invasive approach was feasible in selected patients with colorectal cancer and hepatic metastases. Akiyoshi (24) also published their results following synchronous laparoscopic resection in 10 patients. All primary tumors were located in the sigmoid or rectum. Seven of their patients had an open hepatic resection following their laparoscopic colorectal resection and three patients underwent a minimally invasive resection for an isolated hepatic metastasis. There was no postoperative mortality and one patient developed a complication unrelated to the colorectal or hepatic resection. The open technique required for the hepatic resections limits the significance of this study but provides some insight into the safety of hybrid laparoscopic resections for synchronous colorectal cancer. Lee et al. (25) recently published their 10 patient series of laparoscopic simultaneous colorectal and hepatic resection. Primary tumors were right-sided in four patients, left-sided in three cases, and rectal in three cases. Six patients had single hepatic metastases while the other four patients had ≥2 hepatic metastases. One patient underwent a right hemihepatectomy while others underwent minor hepatic resections. One case required conversion to an open approach due to bleeding from a hepatic vein and this patient also developed an anastomotic leak. There were no postoperative mortalities. This study provides additional limited support for a simultaneous minimally invasive approach for colorectal cancer with limited hepatic metastases. The largest study to date on simultaneous minimally invasive resection of colorectal cancer with hepatic metastases was published by Huh et al. (26). In their study, they compared 20 patients who underwent laparoscopic colorectal resection with 20 patients who had an open approach. In all cases, after the colorectal was completed (either laparoscopically or open), hepatic resection was performed, either laparoscopically or via laparotomy. There were no differences between the laparoscopic and open colectomy groups with regard to the extent of hepatic disease. Minor hepatectomies were performed in 95% of the laparoscopic group and 75% of the open colectomy group. Approximately one third of patients in the laparoscopic colectomy group also had their hepatic resections performed laparoscopically; in all cases minor hepatectomies were performed. No postoperative mortality occurred in either group. Colorectal-related complications were similar between the two groups. One intrahepatic abscess occurred in both the laparoscopic and open group. One patient in the laparoscopic group developed a bile leak. Overall morbidity was similar in the two groups. Despite the small sample size and limited number of patients who underwent a purely minimally invasive approach to both their primary tumor and hepatic metastases, the study by Huh et al. (26) does confirm the general feasibility and safety of a combined minimally invasive approach to colorectal cancer with limited hepatic metastases.
The very limited experience utilizing a completely minimally invasive approach to both a colorectal primary and hepatic metastases prevents us from drawing any major conclusions at this point. However, surgeons who care for patients with synchronous colorectal cancer and hepatic metastases will benefit from the lessons already learned from open synchronous and staged resections. Specifically, surgeons performing each portion of these resections must be able to ensure equivalent safety to that which is associated with open techniques. Furthermore, the literature points to major hepatectomy as the most consistent predictor of postoperative morbidity. Coincident with concerns regarding the safety of a synchronous minimally invasive approach are concerns related to oncologic outcomes. For those few institutions with surgeons with expertise in both minimally invasive colorectal cancer surgery as well as minimally invasive hepatic resection techniques, a minimally invasive simultaneous resection may be considered in patients with limited hepatic disease requiring less than hemihepatectomy.
Conclusion
The current discussion has reviewed the safety and oncologic outcomes associated with simultaneous and staged resections of synchronous metastatic colorectal cancer to the liver. In modern series of simultaneous resections, perioperative mortality is consistently ≤5% but can be expected to be higher when colorectal resections are combined with major hepatectomies involving resection of ≥3 segments. The frequency of complications following synchronous resections involving minor hepatectomies ranges from 5-48% while rates of 33-55% have been reported following major hepatectomies performed simultaneously with colorectal resections. Postoperative morbidity following staged procedures ranges from 16-67% and reflects the fact that major hepatectomies are performed more often in a staged fashion in patients with synchronous colorectal hepatic metastases.
Although complete surgical resection is superior to best systemic therapy in patients with synchronous Stage IV colorectal cancer, no clear benefit has been shown between a simultaneous resection compared to a staged resection. Future studies designed to address this question will have to control for extent of disease, timing and duration of chemotherapy, and chemotherapy-associated hepatotoxicity which may limit the extent of hepatic resection that can be safely performed.
Finally, results following minimally invasive approaches to both the colorectal primary and synchronous hepatic metastases are as yet too preliminary to draw any conclusions regarding the possible advantages of a simultaneous versus staged resection. The same rigorous evaluation of both the safety and oncologic outcomes must be performed before a minimally invasive approach can be adopted.
Footnote
No potential conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 2002;235:759-766. [PubMed]
- Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-825, discussion 825-827. [PubMed]
- Fahy BN, D’Angelica M, DeMatteo RP, et al. Synchronous hepatic metastases from colon cancer: changing treatment strategies and results of surgical intervention. Ann Surg Oncol 2009;16:361-370. [PubMed]
- Brouquet A, Mortenson MM, Vauthey JN, et al. Surgical strategies for synchronous colorectal liver metastases in 156 consecutive patients: classic, combined or reverse strategy? J Am Coll Surg 2010;210:934-941. [PubMed]
- Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 1996;77:1254-1262. [PubMed]
- Sarela AI, Guthrie JA, Seymour MT, Ride E, Guillou PJ, O’Riordain DS. Non-operative management of the primary tumour in patients with incurable stage IV colorectal cancer. Br J Surg 2001;88:1352-1356. [PubMed]
- Tebbutt NC, Norman AR, Cunningham D, et al. Intestinal complications after chemotherapy for patients with unresected primary colorectal cancer and synchronous metastases. Gut 2003;52:568-573. [PubMed]
- Vogt P, Raab R, Ringe B, Pichlmayr R. Resection of synchronous liver metastases from colorectal cancer. World J Surg 1991;15:62-67. [PubMed]
- Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg 2000;191:38-46. [PubMed]
- Jarnagin WR, Gonen M, Fong Y, et al. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg 2002;236:397-406, discussion 406-407. [PubMed]
- Dimick JB, Cowan JA Jr, Knol JA, Upchurch GR Jr. Hepatic resection in the United States: indications, outcomes, and hospital procedural volumes from a nationally representative database. Arch Surg 2003;138:185-191. [PubMed]
- Martin R, Paty P, Fong Y, et al. Simultaneous liver and colorectal resections are safe for synchronous colorectal liver metastasis. J Am Coll Surg 2003;197:233-241, discussion 241-242. [PubMed]
- Tanaka K, Shimada H, Matsuo K, et al. Outcome after simultaneous colorectal and hepatic resection for colorectal cancer with synchronous metastases. Surgery 2004;136:650-659. [PubMed]
- Reddy SK, Pawlik TM, Zorzi D, et al. Simultaneous resections of colorectal cancer and synchronous liver metastases: a multi-institutional analysis. Ann Surg Oncol 2007;14:3481-3491. [PubMed]
- Capussotti L, Ferrero A, Viganò L, Ribero D, Lo Tesoriere R, Polastri R. Major liver resections synchronous with colorectal surgery. Ann Surg Oncol 2007;14:195-201. [PubMed]
- Thelen A, Jonas S, Benckert C, et al. Simultaneous versus staged liver resection of synchronous liver metastases from colorectal cancer. Int J Colorectal Dis 2007;22:1269-1276. [PubMed]
- Martin RC II, Augenstein V, Reuter NP, Scoggins CR, McMasters KM. Simultaneous versus staged resection for synchronous colorectal cancer liver metastases. J Am Coll Surg 2009;208:842-850, discussion 850-852. [PubMed]
- Moug SJ, Smith D, Leen E, Roxburgh C, Horgan PG. Evidence for a synchronous operative approach in the treatment of colorectal cancer with hepatic metastases: a case matched study. Eur J Surg Oncol 2010;36:365-370. [PubMed]
- Emmanouilides C, Sfakiotaki G, Androulakis N, et al. Front-line bevacizumab in combination with oxaliplatin, leucovorin and 5-fluorouracil (FOLFOX) in patients with metastatic colorectal cancer: a multicenter phase II study. BMC Cancer 2007;7:91. [PubMed]
- Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-2059. [PubMed]
- Nguyen KT, Laurent A, Dagher I, et al. Minimally invasive liver resection for metastatic colorectal cancer: a multi-institutional, international report of safety, feasibility, and early outcomes. Ann Surg 2009;250:842-848. [PubMed]
- Kim SH, Lim SB, Ha YH, et al. Laparoscopic-assisted combined colon and liver resection for primary colorectal cancer with synchronous liver metastases: initial experience. World J Surg 2008;32:2701-2706. [PubMed]
- Akiyoshi T, Kuroyanagi H, Saiura A, et al. Simultaneous resection of colorectal cancer and synchronous liver metastases: initial experience of laparoscopy for colorectal cancer resection. Dig Surg 2009;26:471-475. [PubMed]
- Lee JS, Hong HT, Kim JH, et al. Simultaneous laparoscopic resection of primary colorectal cancer and metastatic liver tumor: initial experience of single institute. J Laparoendosc Adv Surg Tech A 2010;20:683-687. [PubMed]
- Huh JW, Koh YS, Kim HR, Cho CK, Kim YJ. Comparison of laparoscopic and open colorectal resections for patients undergoing simultaneous R0 resection for liver metastases. Surg Endosc 2011;25:193-198. [PubMed]


