
Radiation biology considerations of proton therapy for gastrointestinal cancers
Clinical enthusiasm for proton therapy (PT) is high, with an exponential increase in the number of centers offering treatment (1). Attraction for this charged particle therapy modality stems from the favorable proton dose distribution, with low radiation dose absorption on entry and maximum radiation deposition at the Bragg peak (2). This leads to improved tumor targeting and normal tissue sparing and is particularly advantageous for the treatment of tumors positioned near crucial structures (3). The proton radiation track structure on the nanoscale and hence energy deposition differs from that of conventional photon radiation therapy (RT), leading to a different biological effect. DNA damage generated by particle tracks with high linear energy transfer (LET) are thought to be more complex and more difficult to repair (4,5).
The current clinical convention is to use a fixed relative biological effectiveness (RBE) value of 1.1 in order to correct the physical dose relative to photon therapy (i.e., proton radiation is 10% more biologically effective then photon radiation) (6). RBE is defined as the ratio of physical doses that cause the same biological effect and is calculated as RBE =Dcontrol ⁄Dtest. In this equation Dcontrol is the physical dose of a reference radiation modality (X-rays) and Dtest is the physical dose of the radiation modality being investigated (protons).
In recent years, concerns about the potential side effects of PT have emerged. Various studies (7-17) and review articles (18-22) have sought to better quantify the RBE of PT and shine some light on the complexity of this problem. In a 2014 review article, Paganetti showed the average proton RBE values across a spread out Bragg peak (SOBP) range from 0.9–1.7 (19). A large body of in-vitro evidence for the variation in PT RBE has been published and collected in the online, open-access Particle Irradiation Data Ensemble (PIDE) database (23).
The use of a constant RBE disregards the experimental evidence demonstrating that RBE has a complex dependency on dose per fraction, tissue type, LET, and biological endpoint (4). This translates to an uncertainty in the biological effective dose delivered to the patient, more so in the regions surrounding the Bragg peak where the RBE is hypothesized to be significantly higher, and may lead to substantial dose increases to normal tissue.
Based on the in vitro data, many RBE models that relate LET and RBE have been developed (24-30). Figure 1 represents a simple linear model between RBE and LET (24,31). Most of the models have similar features, with the major difference in the magnitude of the RBE at the distal falloff. One major item the in-vitro studies do not include is the effect of fractionation. There is evidence to suggest that with fractionation, the RBE of the high LET region may be amplified (32). Figure 2 shows that potentially high biological dose can exist in critical normal tissue and with slight modification to the overall dose distribution; these biological hot spots can be reduced.
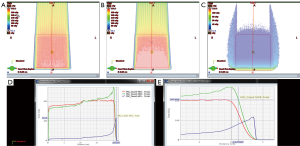
Reduction in biologic hot spots of non-target tissue is paramount in proton RT planning as the primary benefit of proton RT is a reduction in organ at risk (OAR) irradiation. New and emerging clinical data is in support of variable proton biological effectiveness and demonstrate late toxicity, presumably associated with high biological dose, to OAR (33-39). In some situations, such as pediatric brain cancer, this has prompted expert working groups for consensus guidelines on proton planning to account for this (40-42).
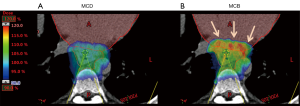
With conventional RT planning, target expansions are created to account for subclinical disease, target motion, and variations in treatment set up. Often such expansions may result in target volumes extending to or within adjacent organs. The Bragg peak may in fact be within an OAR, with a higher biologic dose in OAR than tumor. This is highlighted in several examples of malignancies within the gastrointestinal (GI) tract.
For example, as illustrated in Figure 2, several cardiac structures, including the left atrium, left ventricle, and coronary arteries, may be situated just anterior to or within the radiation target volume for a distal esophageal cancer. Without considering the biologic effective dose during proton planning, proton RT may increase toxic effects to the heart (Figure 2B). Thus, despite reduction in integral heart dose, which is one of the primary considerations of PT for esophagus cancer, a proton plan not accounting for biologic effective dose may increase toxicity. Predictive modeling of biologic dose builds up and focal reduction in physical dose or reduction in the overall dose and fractionation are methods to reduce potential toxicity from biologic hotspots.
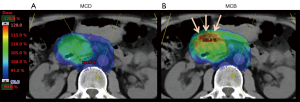
Similar to esophagus cancer, most abdominal cancers are situated in tight proximity to OAR. For cancers of the pancreas head, the duodenum, large bowel and pylorus of the stomach are adjacent OAR and may be located within the distal Bragg peak when designing a proton plan with posterior fields (Figure 3). Increase in biological effective dose in these organs may result in a higher risk of radiation enteritis, ulceration and/or hemorrhage, and possible perforation. In most cases, this can be accounted for with slight modifications to the physical dose or treating to a lower total physical dose. In some cases, it is beneficial to add an additional field, despite increasing integral body dose, to decrease the overall biological effective dose.
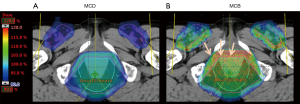
In the management of anal cancer, PT may reduce substantial dose to adjacent perineum, genitals, and groin thereby reducing risk such as dermatitis and long-term sexual dysfunction. Figure 4 shows a typical proton plan with bilateral posterior oblique and anterior fields to provide dose to the pelvis and inguinal lymph nodes. The end of range biologic dose builds up in the anterior bladder, urethra and obturator tissue. Thus, while sparing perineal tissue and genitalia, the enhanced biologic dose may increase urethral or other genitourinary toxicity relative to other forms of RT.
Certain planning techniques are often useful to control OAR position or limit target motion and volume. Often these are simple, reproducible measures such as breath hold, controlled urination and bladder filling, defecation, or fasting before treatment. A patient’s nutritional status may change during the course of treatment resulting in external or internal contour changes and impact on the position of the distal Bragg peak. Additional imaging and verification of proton dose is critical to ensure proper delivery of protons during the course of treatment with adaptive re-planning as needed.
Overall, PT has promise to treat many cancer sites with similar efficacy as conventional RT but with fewer acute and late toxicities. However, further knowledge of biologic effective dose and its impact on both cancer and adjacent OAR is paramount for effective and safe treatment of patients with PT. With greater understanding of the biological effect, there may be a future role for purposefully increasing this effect within the target for radiation resistant tumors.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Girdhani S, Sachs R, Hlatky L. Biological effects of proton radiation: an update. Radiat Prot Dosimetry 2015;166:334-8. [Crossref] [PubMed]
- Lühr A, von Neubeck C, Krause M, et al. Relative biological effectiveness in proton beam therapy - Current knowledge and future challenges. Clin Transl Radiat Oncol 2018;9:35-41. [Crossref] [PubMed]
- Ilicic K, Combs SE, Schmid TE. New insights in the relative radiobiological effectiveness of proton irradiation. Radiat Oncol 2018;13:6. [Crossref] [PubMed]
- Durante M. New challenges in high-energy particle radiobiology. Br J Radiol 2014;87:20130626. [Crossref] [PubMed]
- Trofimov AV, Craft D, Unkelbach J. Treatment-Planning Optimization. In: Paganetti H. editor. Proton therapy physics. Boca Raton: CRC Press, 2012.
- Paganetti H, van Luijk P. Biological considerations when comparing proton therapy with photon therapy. Semin Radiat Oncol 2013;23:77-87. [Crossref] [PubMed]
- Ando K, Furusawa Y, Suzuki M, et al. Relative biological effectiveness of the 235 MeV proton beams at the National Cancer Center Hospital East. J Radiat Res 2001;42:79-89. [Crossref] [PubMed]
- Belli M, Bettega D, Calzolari P, et al. Inactivation of human normal and tumour cells irradiated with low energy protons. Int J Radiat Biol 2000;76:831-9. [Crossref] [PubMed]
- Belli M, Cherubini R, Finotto S, et al. RBE-LET relationship for the survival of V79 cells irradiated with low energy protons. Int J Radiat Biol 1989;55:93-104. [Crossref] [PubMed]
- Britten RA, Nazaryan V, Davis LK, et al. Variations in the RBE for cell killing along the depth-dose profile of a modulated proton therapy beam. Radiat Res 2013;179:21-8. [Crossref] [PubMed]
- Calugaru V, Nauraye C, Noel G, et al. Radiobiological characterization of two therapeutic proton beams with different initial energy spectra used at the Institut Curie Proton Therapy Center in Orsay. Int J Radiat Oncol Biol Phys 2011;81:1136-43. [Crossref] [PubMed]
- Chaudhary P, Marshall TI, Perozziello FM, et al. Relative biological effectiveness variation along monoenergetic and modulated Bragg peaks of a 62-MeV therapeutic proton beam: a preclinical assessment. Int J Radiat Oncol Biol Phys 2014;90:27-35. [Crossref] [PubMed]
- Guan F, Bronk L, Titt U, et al. Spatial mapping of the biologic effectiveness of scanned particle beams: towards biologically optimized particle therapy. Sci Rep 2015;5:9850. [Crossref] [PubMed]
- Maeda K, Yasui H, Matsuura T, et al. Evaluation of the relative biological effectiveness of spot-scanning proton irradiation in vitro. J Radiat Res 2016;57:307-11. [Crossref] [PubMed]
- Marshall TI, Chaudhary P, Michaelidesova A, et al. Investigating the Implications of a Variable RBE on Proton Dose Fractionation Across a Clinical Pencil Beam Scanned Spread-Out Bragg Peak. Int J Radiat Oncol Biol Phys 2016;95:70-7. [Crossref] [PubMed]
- Matsumoto Y, Matsuura T, Wada M, et al. Enhanced radiobiological effects at the distal end of a clinical proton beam: in vitro study. J Radiat Res 2014;55:816-22. [Crossref] [PubMed]
- Tang JT, Inoue T, Inoue T, et al. Comparison of radiobiological effective depths in 65-MeV modulated proton beams. Br J Cancer 1997;76:220-5. [Crossref] [PubMed]
- Gerweck LE, Kozin SV. Relative biological effectiveness of proton beams in clinical therapy. Radiother Oncol 1999;50:135-42. [Crossref] [PubMed]
- Paganetti H. Relative biological effectiveness (RBE) values for proton beam therapy. Variations as a function of biological endpoint, dose, and linear energy transfer. Phys Med Biol 2014;59:R419-72. [Crossref] [PubMed]
- Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407-21. [Crossref] [PubMed]
- Tommasino F, Durante M. Proton radiobiology. Cancers (Basel) 2015;7:353-81. [Crossref] [PubMed]
- Yang TC. Proton radiobiology and uncertainties. Radiat Meas 1999;30:383-92. [Crossref] [PubMed]
- Friedrich T, Grun R, Scholz U, et al. Sensitivity analysis of the relative biological effectiveness predicted by the local effect model. Phys Med Biol 2013;58:6827-49. [Crossref] [PubMed]
- Beltran C, Tseung HWC, Augustine KE, et al. Clinical Implementation of a Proton Dose Verification System Utilizing a GPU Accelerated Monte Carlo Engine. Int J Part Ther 2016;3:312-9. [Crossref] [PubMed]
- Carabe A, Moteabbed M, Depauw N, et al. Range uncertainty in proton therapy due to variable biological effectiveness. Phys Med Biol 2012;57:1159-72. [Crossref] [PubMed]
- Frese MC, Yu VK, Stewart RD, et al. A mechanism-based approach to predict the relative biological effectiveness of protons and carbon ions in radiation therapy. Int J Radiat Oncol Biol Phys 2012;83:442-50. [Crossref] [PubMed]
- McMahon SJ, Paganetti H, Prise KM. LET-weighted doses effectively reduce biological variability in proton radiotherapy planning. Phys Med Biol 2018;63:225009. [Crossref] [PubMed]
- McNamara AL, Schuemann J, Paganetti H. A phenomenological relative biological effectiveness (RBE) model for proton therapy based on all published in vitro cell survival data. Phys Med Biol 2015;60:8399-416. [Crossref] [PubMed]
- Wan Chan Tseung HS, Ma J, Kreofsky CR, et al. Clinically Applicable Monte Carlo-based Biological Dose Optimization for the Treatment of Head and Neck Cancers With Spot-Scanning Proton Therapy. Int J Radiat Oncol Biol Phys 2016;95:1535-43. [Crossref] [PubMed]
- Wedenberg M, Lind BK, Hardemark B. A model for the relative biological effectiveness of protons: the tissue specific parameter alpha/beta of photons is a predictor for the sensitivity to LET changes. Acta Oncol 2013;52:580-8. [Crossref] [PubMed]
- Fossum CC, Beltran CJ, Whitaker TJ, et al. Biological Model for Predicting Toxicity in Head and Neck Cancer Patients Receiving Proton Therapy. Int J Part Ther 2017;4:18-25. [Crossref] [PubMed]
- Karger CP, Peschke P, Sanchez-Brandelik R, et al. Radiation tolerance of the rat spinal cord after 6 and 18 fractions of photons and carbon ions: experimental results and clinical implications. Int J Radiat Oncol Biol Phys 2006;66:1488-97. [Crossref] [PubMed]
- Schlampp I, Karger CP, Jakel O, et al. Temporal lobe reactions after radiotherapy with carbon ions: incidence and estimation of the relative biological effectiveness by the local effect model. Int J Radiat Oncol Biol Phys 2011;80:815-23. [Crossref] [PubMed]
- Gillmann C, Lomax AJ, Weber DC, et al. Dose-response curves for MRI-detected radiation-induced temporal lobe reactions in patients after proton and carbon ion therapy: Does the same RBE-weighted dose lead to the same biological effect? Radiother Oncol 2018;128:109-14. [Crossref] [PubMed]
- Peeler CR, Mirkovic D, Titt U, et al. Clinical evidence of variable proton biological effectiveness in pediatric patients treated for ependymoma. Radiother Oncol 2016;121:395-401. [Crossref] [PubMed]
- Gillmann C, Jakel O, Schlampp I, et al. Temporal lobe reactions after carbon ion radiation therapy: comparison of relative biological effectiveness-weighted tolerance doses predicted by local effect models I and IV. Int J Radiat Oncol Biol Phys 2014;88:1136-41. [Crossref] [PubMed]
- Pehlivan B, Ares C, Lomax AJ, et al. Temporal lobe toxicity analysis after proton radiation therapy for skull base tumors. Int J Radiat Oncol Biol Phys 2012;83:1432-40. [Crossref] [PubMed]
- Underwood TSA, Grassberger C, Bass R, et al. Asymptomatic Late-phase Radiographic Changes Among Chest-Wall Patients Are Associated With a Proton RBE Exceeding 1.1. Int J Radiat Oncol Biol Phys 2018;101:809-19. [Crossref] [PubMed]
- Giantsoudi D, Sethi RV, Yeap BY, et al. Incidence of CNS Injury for a Cohort of 111 Patients Treated With Proton Therapy for Medulloblastoma: LET and RBE Associations for Areas of Injury. Int J Radiat Oncol Biol Phys 2016;95:287-96. [Crossref] [PubMed]
- Giantsoudi D, Adams J, MacDonald SM, et al. Proton Treatment Techniques for Posterior Fossa Tumors: Consequences for Linear Energy Transfer and Dose-Volume Parameters for the Brainstem and Organs at Risk. Int J Radiat Oncol Biol Phys 2017;97:401-10. [Crossref] [PubMed]
- Gentile MS, Yeap BY, Paganetti H, et al. Brainstem Injury in Pediatric Patients With Posterior Fossa Tumors Treated With Proton Beam Therapy and Associated Dosimetric Factors. Int J Radiat Oncol Biol Phys 2018;100:719-29. [Crossref] [PubMed]
- Haas-Kogan D, Indelicato D, Paganetti H, et al. National Cancer Institute Workshop on Proton Therapy for Children: Considerations Regarding Brainstem Injury. Int J Radiat Oncol Biol Phys 2018;101:152-68. [Crossref] [PubMed]


