Bone marrow infiltration as the initial presentation of gastric signet ring cell adenocarcinoma
Case presentation
A 52-year-old African American man, with a history of sickle cell trait, alpha-thalassemia, gynecomastia and nephrolithiasis presented to the emergency department of an urban, tertiary-care medical center with worsening back pain. The patient stated that 3 weeks prior he experienced minor trauma while driving a bus and has since had severe pain in his lumbar region that was unresponsive to over-the-counter analgesics. The patient denied further symptoms, including weight loss, fever, night sweats, abdominal pain, dyspepsia, nausea, vomiting, hematochezia, and melena. Of note, the patient had a family history significant for a first-degree relative with multiple myeloma (MM) and another with breast cancer. On physical exam, the patient was tender to palpation in the lower thoracic and lumbar spine. The patient had no saddle anesthesia or focal neurologic deficits, and the abdominal exam was benign. Initial laboratory evaluation revealed a white blood cell (WBC) count of 13.1 K/UL, a hemoglobin of 10.2 G/DL with an MCV of 65, and a platelet count of 184 K/UL. The creatinine was elevated to 1.47 mg/DL and the serum calcium was 10.8 mg/DL. Liver function tests (LFT) were significant for an alkaline phosphatase of 1,372 U/L, while other LFTs were normal. Computed tomography imaging of the chest, abdomen, and pelvis revealed a compression fracture of the L1 vertebral body as well as lytic lucencies in the T5 and T9 vertebral bodies (Figure 1), prominent lymph nodes in the right upper quadrant, and a 1.8 cm hypoattenuating lesion in the left lobe of the thyroid gland.

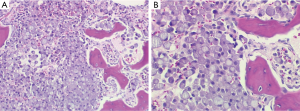
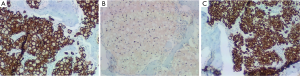
The patient was admitted to the medical ward for further evaluation of the lytic lesions, as well as pain management. The presenting labwork, lytic lesions, and family history prompted an initial evaluation for MM. A serum protein electrophoresis, serum immunofixation, and serum free light chain assay returned positive for a monoclonal IgG kappa paraprotein. A subsequent bone marrow evaluation did not yield an aspirate (“dry tap”), but a biopsy specimen was obtained. The pathologic review of this specimen was significant for marked hypercellularity (90%), which was due to an extensive infiltrate of signet ring cells accounting for 90% of the hypercellularity (Figure 2). Myeloid and erythroid elements were markedly decreased and megakaryocytes were nearly absent. A mild plasmacytosis was present with small pockets of mature plasma cells. Immunohistochemistry staining of the signet ring cells was positive for PAS, AE1/AE3, Cam5.2 (Figure 3A), CD138, and cyclin D1. Staining with CD×2 was positive, indicating a gastrointestinal origin for the signet ring cells (Figure 3B). Subsequent staining with CK7 was positive (Figure 3C), while CK20 was rarely positive. The final pathology was metastatic signet ring cell carcinoma of gastrointestinal primary, most likely upper gastrointestinal.
The findings described above prompted a workup for a gastrointestinal malignancy. Endoscopy of the upper gastrointestinal tract revealed a malignant-appearing mass in the gastric pylorus. Biopsy was positive for signet ring cell carcinoma. Helicobacter pylori testing via Giemsa stain was negative. The patient was subsequently discharged, and was seen in the oncology clinic to begin chemotherapy for metastatic gastric cancer with epirubicin, oxaliplatin, and capecitabine (EOX).
Discussion
Gastric cancer accounts for a significant worldwide cancer burden. The WHO estimates there were 952,000 new cases of gastric cancer worldwide in 2012 and 723,000 deaths (1). In the United States, the cancer burden from gastric malignancy is less than other parts of the world, but it is still significant, with an estimated 22,220 new cases in 2014 and 10,990 deaths (2).
Gastric cancer trends have undergone profound changes since the turn of the century. In the US, gastric cancer was the leading cause of cancer death in men in 1930. Currently, gastric cancer is the twelfth leading cause of cancer death in men (2). Underlying this decline in mortality is a continuous decrease in gastric cancer incidence since the early 20th century. Changes in certain environmental and societal factors have led to a decrease in the predominant, intestinal-type gastric cancer. The major factors include reduced prevalence of Helicobacter pylori infection, decreased consumption of salted fish and meats, decreased consumption of smoked foods, and a decline in the use of tobacco products. In contrast, the diffuse type of gastric cancer has been increasing over the past 30 years (3).
Gastric cancer has been broadly divided into intestinal-type and diffuse-type since this was initially described almost 50 years ago (4). The histologic shift from intestinal type gastric cancer to diffuse type will have important clinical implications. Diffuse type gastric cancer occurs at a younger age, and is more advanced at presentation, particularly when compared to well or moderately differentiated intestinal type gastric cancer (5). The traditional risk factors for stomach cancer do not play as great a role in diffuse type gastric cancer, and studies have described the importance of familial syndromes such as hereditary diffuse gastric cancer (6). This is of particular importance to our case because our patient had the signet ring cell type of gastric adenocarcinoma, which is a type of diffuse gastric cancer. Our patient presents with younger age, which is a hallmark of the diffuse type, as was our patient’s advanced disease at diagnosis. The unique characteristic of our case is that our patient presented with only back pain, denying all gastrointestinal complaints. It was through the evaluation of our patient’s back pain that the patient was ultimately diagnosed with signet ring cell gastric adenocarcinoma.
The occurrence of bone marrow metastasis has been documented in gastric cancer, although this is rarely the initial presentation. If bone marrow involvement is discovered, it is usually during the workup for metastatic disease. Interestingly, our patient only had mild microcytic anemia, which was likely preceding his gastric cancer and related to his alpha-thalassemia. Our patient did not have leukopenia or thrombocytopenia, the latter of which was determined to be indicative of bone marrow involvement in at least one study (7). When bone marrow metastasis occurs, it is more commonly a signet ring cell subtype of gastric carcinoma and occurs in younger patients (8). The prognosis for bone marrow involvement with gastric adenocarcinoma is abysmal, with patient’s living an average of 44 days from the time of documented bone marrow involvement (7).
This case is significant for two reasons. First, it highlights the importance of a broad differential diagnosis when approaching a patient with lytic bone lesions. Second, bone marrow involvement is more common in patients with diffuse type gastric adenocarcinoma and occurs in particularly young patients. The increasing incidence of diffuse type gastric adenocarcinoma means bone marrow metastases will likely play a greater role in the presentation and management of gastric cancer.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available online: http://globocan.iarc.fr, accessed on 2/12/2014.
- American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society, 2014.
- Henson DE, Dittus C, Younes M, et al. Differential trends in the intestinal and diffuse types of gastric carcinoma in the United States, 1973-2000: increase in the signet ring cell type. Arch Pathol Lab Med 2004;128:765-70. [PubMed]
- Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand 1965;64:31-49. [PubMed]
- Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol 2014;21:1678-85. [PubMed]
- Blair V, Martin I, Shaw D, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol 2006;4:262-75. [PubMed]
- Kim HS, Yi SY, Jun HJ, et al. Clinical outcome of gastric cancer patients with bone marrow metastases. Oncology 2007;73:192-7. [PubMed]
- Kusumoto H, Haraguchi M, Nozuka Y, et al. Characteristic features of disseminated carcinomatosis of the bone marrow due to gastric cancer: the pathogenesis of bone destruction. Oncol Rep 2006;16:735-40. [PubMed]


