
Long-term outcomes by response to neoadjuvant chemotherapy or chemoradiation in patients with resected pancreatic adenocarcinoma
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a lethal, yet relatively common malignancy, with over 55,000 patients diagnosed annually (1). Surgical resection is the only potential curative treatment option; however, only 15–20% of patients are considered acceptable candidates for surgery (2). Even following complete resection, prognosis remains poor, with 5-year estimated survival rates of 10% and 30% in cases of node-positive and node-negative disease, respectively (3,4).
While multiple prospective studies have supported adjuvant systemic therapy for these patients, the data regarding neoadjuvant therapy are comparatively sparse (5-12). Current guidelines recommend consideration of neoadjuvant therapy in patients with borderline resectable (BR) disease. For patients with BR disease, neoadjuvant therapy may increase the rate of R0 resection (10). This is particularly important in the context of PDAC since survival following R1/2 resection is comparable to that of unresected disease (13,14). Additionally, roughly 30% of patients with initially unresectable disease can be converted to resectable disease after neoadjuvant therapy (15,16).
An additional advantage to neoadjuvant therapy relates to the assessment of clinical response (tumor biology) and individualizing subsequent therapy accordingly. Following neoadjuvant chemotherapy (nCT) or chemoradiotherapy (nCRT), a meta-analysis using the response evaluation criteria in solid tumors (RECIST) showed that a partial response was achieved in 29%, stable disease in 46%, progression in 17%, and a complete response in 3% (17). Despite these data, long-term outcomes based on response to neoadjuvant therapy in PDAC have not been well evaluated thus far. Analogous investigations of other neoplasms have proven highly useful to quantitatively describe expected outcomes based on clinical response to neoadjuvant therapy (18,19). Given this knowledge gap, our aim was to evaluate PDAC outcomes based on response to neoadjuvant therapy (and predictive factors thereof) through analysis of a large contemporary database.
Methods
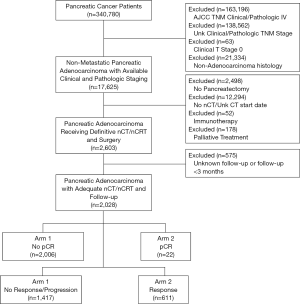
The National Cancer Database (NCDB) is a tumor registry overseen by the American Cancer Society and the American College of Surgeons. It contains de-identified data involving approximately 70% of cancer cases in the United States from over 1,500 hospitals accredited by the Commission on Cancer (CoC) and is thus exempt from institutional review board supervision. We queried the database [2004–2015] to identify patients with newly diagnosed PDAC who had received nCT or nCRT. Patients were excluded from the study if any of the following criteria were met: non-adenocarcinoma histology, unknown clinical or pathological stage (in order to allow for response assessment), stage IV disease, clinical T0 or TX (evidence of a primary tumor was needed to assess for response), lack of pancreatectomy, and receipt of immunotherapy or palliative treatment (as characterized by the NCDB, this includes therapy intended to control symptoms, alleviate pain and make the patient more comfortable). Patients with follow-up less than one month were also excluded to account for immortal time bias. Patients who had received adjuvant therapy were included in this study as this does not impact pathologic response. A complete CONSORT diagram depicting this selection process is outlined in Figure S1.
To evaluate response to neoadjuvant therapy, clinical T and N stage was compared to post-nCT/nCRT pathologic T and N stage (American Joint Cancer Committee, 7th edition); this was done by means of evaluating cT (designated as “a”) to ypT (“b”) disease, and cN (“c”) to ypN (“d”) disease, similar to prior investigations in other disease sites (18,19). Based on these comparisons, patients were categorized into three cohorts. Responders referred to a≥b (primary tumor response) and/or c≥d (nodal response) (with the exception of a=b & c=d). Progressors were defined by a<b & c=d (tumor progression), a=b & c<d (nodal progression), or a<b & c<d (tumor and nodal progression). Non-responders encompassed both a lack of response (a=b & c=d) and mixed response (a>b & c<d, or a<b & c>d). In the context of the above response schema, it is noteworthy that the NCDB does not code for imaging-based response criteria, such as RECIST.
Data were analyzed using Medcalc Version 18 (Ostend, Belgium). Overall survival (OS) was calculated in months from time of diagnosis to time of death (or censored at last contact). The Kaplan-Meier method was utilized to estimate survival over time; reverse Kaplan-Meier survival analysis was used to estimate median follow-up time. Cox proportional hazards model was used for multivariable survival analysis. Adjusted hazard ratios and 95% confidence intervals were reported, using an α-level of 0.05 to indicate statistical significance.
Results
Patient and disease characteristics
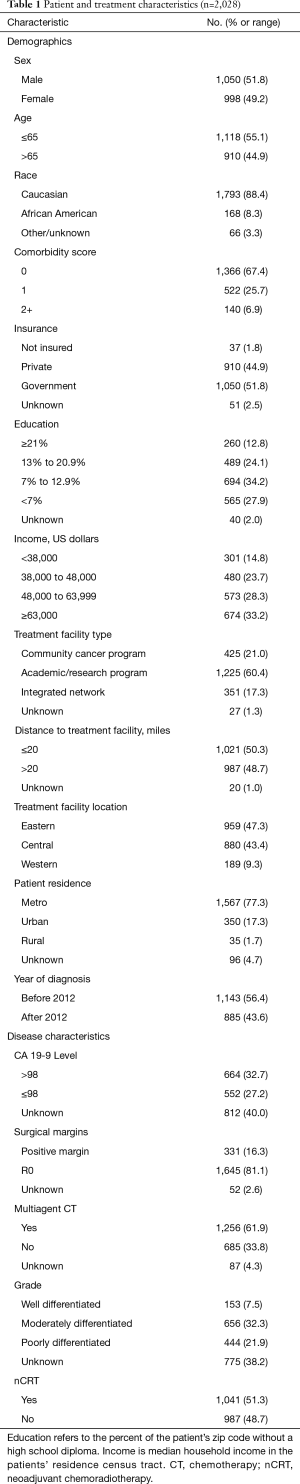
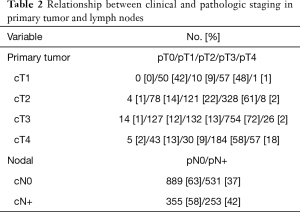
We identified 2,028 patients meeting the above eligibility criteria, with 30% of patients (n=611) having a response to nCT/nCRT, 32% (n=640) progressing, and 38% (n=777) having no response. Twenty-two patients (1%) experienced pathologic complete response (pCR). Table 1 provides baseline characteristics for the entire cohort. The majority of patients were cT3 (52%) and cN0 (70%). Nearly two-thirds of the cohort (65%) received multiagent nCT, while approximately half (51%) received neoadjuvant radiation therapy. Following surgery, more than 80% of patients had negative margins (R0 resection). Table 2 compares the clinical and pathologic staging of both the primary tumor and lymph nodes for the entire cohort.
Full table
Full table
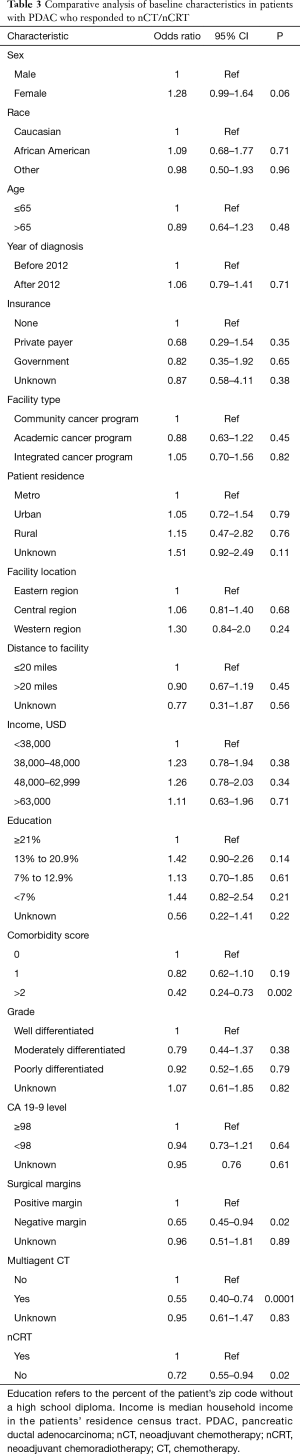
Differences in demographic and disease-related characteristics between those patients who responded to nCR/nCRT and those who did not are outlined in Table 3. Of note, receipt of multiagent nCT [P<0.001; hazard ratio (HR): 0.55; 95% confidence interval (CI): 0.40–0.74] and neoadjuvant radiation therapy (P=0.02; HR: 0.72; 95% CI: 0.55–0.94) were associated with higher likelihood of pathologic response. Additionally, responding patients were more likely to have negative surgical margins (P=0.02; HR: 0.65; 95% CI: 0.45–0.94). Examining the 22 pCR patients, only receipt of neoadjuvant radiation therapy predicted for pCR (P=0.05).
Full table
Survival
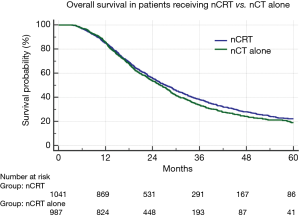
Median follow-up for the full cohort was 49 months (95% CI: 44–49 months). Median OS was assessed by treatment response (29.9 months for responders, 24.3 months for non-responders, and 22.2 months for progressors); OS was higher among responders compared to either non-responders (P<0.001; HR: 0.63; 95% CI: 0.57–0.71) or progressors (P<0.001; HR: 0.52; 95% CI: 0.46–0.59) (Figure 1A). Additionally, the average OS for patients with pCR versus all non-pCR responders, non-responders and progressors was 55.5 vs. 26.6 months (median OS of the pCR cohort was not reached) (P=0.001) (Figure 1B). To evaluate the comparative effect of primary tumor versus nodal response, responders were further subdivided into those experiencing a T response, an N response, or both. This revealed similar OS between each subgroup (29.5 vs. 28.6 vs. 35.4 months, respectively) (P=0.28) (Figure 1C). When comparing OS between those patients receiving nCRT vs. nCT, there was no significant difference in OS (Figure 2).
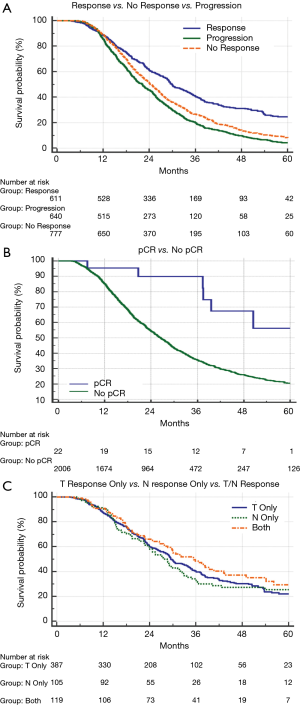
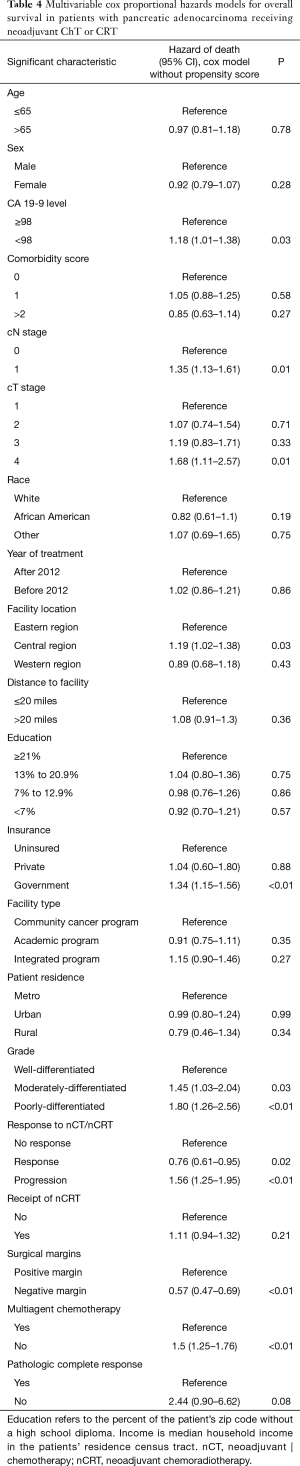
On multivariable analysis, treatment response was independently associated with OS. Progression following treatment was independently associated with decreased OS (P<0.05 for both; Table 4). Additionally, R0 resection, receipt of multiagent chemotherapy, and lower CA 19-9 level also predicted for better OS (P<0.05 for all). Although limited by the small sample sizes, pCR showed a trend toward increased OS (HR 0.41 with non-pCR as a reference, P=0.08).
Full table
Discussion
Response of PDAC to neoadjuvant therapy influences prognosis, but to date high-volume data of long-term outcomes based on treatment response have been lacking. Our results have shown that following nCT/nCRT, T/N response was achieved in 30% of patients, compared to progression in 32% and non-response in 38%. The pCR rate is this study was 1%. Treatment response significantly influenced OS, including a strong trend for pCR (P=0.08) (Table 4).
Several studies have assessed the impact of nCT regimens on oncologic outcomes in the resectable or BR populations (12,20,21). A recent phase II trial evaluated R0 resection rates in patients with previously untreated, BR PDAC following neoadjuvant FOLFIRINOX. R0 resection was achieved in 65% of patients, and median progression free survival (PFS) and OS were found to be prolonged (20). The current PREOPANC trial has shown similar results, with preoperative chemoradiotherapy significantly prolonging OS in patients with BR PDAC when compared with immediate surgery (12). A recent meta-analysis aimed to clarify the effectiveness of FOLFIRINOX as part of a neoadjuvant regimen when compared with single-agent gemcitabine. The median OS ranged from 16–38 months; previous studies of single-agent gemcitabine had observed a median OS of only 6–13 months, indicating that FOLFIRINOX may be more efficacious as a neoadjuvant regimen (21). Taken together, these results support a multiagent neoadjuvant regimen in these patients, as it could increase R0 resection rates and lengthen OS. Our results support these findings, as receipt of multiagent chemotherapy was associated with improved survival. While combination chemotherapy may be more effective in killing tumor cells, the toxicity associated with multiple agents remains a potentially inhibitory risk. Clinical trials are ongoing to determine whether FOLFIRINOX is more effective than gemcitabine/nab-paclitaxel as neoadjuvant therapy (NCT02562716) (22).
The addition of RT to nCT remains controversial. Our data demonstrate an association between nCRT and a higher rate of downstaging (including nodal sterilization), potentially impacting OS indirectly. This concept parallels data from non-small cell lung cancer (23,24). Among PDAC patients, recent retrospective data showed that when used as part of a neoadjuvant regimen with either single agent or multiagent chemotherapy, use of nRT resulted in a significantly higher likelihood of nodal downstaging. Moreover, patients with node-negative status following neoadjuvant therapy had a significantly lower risk of death as compared to node-positive cases (25). Our results demonstrate that patients with a response to neoadjuvant therapy are more likely to have R0 resection. Taken together, these results suggest that while neoadjuvant CRT may not directly play a role in increasing OS, it could increase the likelihood of developing a tumor response and make surgery more effective. Lastly, the improved distant control from new multi-agent chemotherapy regimens may shift patterns of failure, implying a greater necessity for local control, which can be better addressed with RT (26).
pCR in PDAC is an extremely rare occurrence, seen in only 3–11% of patients who have undergone resection after receiving neoadjuvant treatment (27), consistent with our results. It should be noted that the rarity of pCR makes interpreting results related to OS difficult. Notably, when multi-agent chemotherapeutic regimens such as FOLFIRINOX are used, however, the rate of pCR has been reported as high as 13% (28). When pCR is achieved, the recurrence risk is sharply lower than expected, thus resulting in improved survival (27). A retrospective study from Johns Hopkins University attempted to clarify the relationship between OS and pCR in 186 patients with PDAC who received neoadjuvant chemoradiotherapy (nCRT) followed by pancreatectomy. The median disease-free survival and OS were found to be significantly increased in patients with pCR when compared to those with near complete response (defined as a primary tumor less than 1cm without nodal metastasis) at 26 vs. 12 months and 60 vs. 26 months, respectively (29).
Regarding the limitations of this study, there are factors inherent to the NCDB which must be considered when interpreting these results (30-44), in addition to inevitable retrospective selection biases. Most importantly, the results of our study depend on the accuracy of preoperative clinical staging, which is not specified in the NCDB. If cT and cN staging was inaccurate in the NCDB, our grouping of responders, non-responders, and progressors (and survival results thereof) could be affected. The NCDB does not code for RECIST response, necessitating comparison of cT/N to ypT/N to evaluate clinical response [similar to existing studies (18,19)], which may be less clinically significant in some instances (e.g., a 2.1 cm cT2 tumor to a 1.9 cm pT1 tumor). Similarly, as ycN staging is not coded for in the NCDB, our comparison depends on radiologic diagnosis of lymph node metastases, which may not always be accurate. Furthermore, it is unknown if patients underwent pancreatic protocol CT evaluation, which theoretically may yield higher diagnostic accuracy in evaluating the local extent of disease. However, the rates of response (or lack thereof) were roughly similar to studies using RECIST (17). Second, the NCDB lacks data regarding specific chemotherapeutic agents and number of cycles completed, characteristics of RT regimens (e.g., target volumes), tumor biology, performance status, and salvage therapies. Third, the NCDB does not provide data on whether patients are resectable, BR or locally advanced, meaning that translating our results to these specific subgroups of patients is challenging. Fourth, the NCDB also does not code for the time from nC(R)T completion to surgery. Fifth, CA 19-9 and tumor grade were not reported in approximately 40% of patients, limiting robust assessment thereof. As noted above, with such a small sample size, interpreting our results related to pCR is challenging. Furthermore, patients receiving adjuvant therapy were included in the study such that some patients may have received both neoadjuvant and adjuvant therapy which may have affected survival data. Additionally, the NCDB does not provide detailed information regarding surgical resection or vascular invasion, so this data could not be included in our analysis. Lastly, although the NCDB includes data for 70% of the United States population, only CoC-accredited facilities contribute data; as such, these findings may not necessarily be representative of the entire United States population.
In summary, we have shown that receipt of multiagent nCT and nCRT predicts for a downstaging response, and in patients with a response, there is a significant increase in OS. Our novel data thus suggests that more aggressive neoadjuvant therapy can lead to improved outcomes in PDAC patients.
Acknowledgments
The authors would like to thank the Department of Internal Medicine, the Department of Medical Oncology and the Department of Radiation Oncology at Allegheny Health Network for their assistance in the production of this project.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Ko CY, et al. National failure to operate on early stage pancreatic cancer. Ann Surg 2007;246:173-80. [Crossref] [PubMed]
- Kang MJ, Jang JY, Chang YR, et al. Revisiting the concept of lymph node metastases of pancreatic head cancer: Number of metastatic lymph nodes and lymph node ratio according to N stage. Ann Surg Oncol 2014;21:1545-51. [Crossref] [PubMed]
- Allen PJ, Kuk D, Castillo CFD, et al. Multi-institutional validation study of the american joint commission on cancer (8th Edition) changes for T and N staging in patients with pancreatic adenocarcinoma. Ann Surg 2017;265:185-91.
- Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs. gemcitabine following pancreatic cancer resection. JAMA 2010;304:1073. [Crossref] [PubMed]
- Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med 2004;350:1200-10. [Crossref] [PubMed]
- Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs. gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma. JAMA 2008;299:1019. [Crossref] [PubMed]
- Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs. observation in patients undergoing curative-intent resection of pancreatic cancer. JAMA 2007;297:267. [Crossref] [PubMed]
- Oettle H, Neuhaus P, Hochhaus A, et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer. JAMA 2013;310:1473. [Crossref] [PubMed]
- National Comprehensive Cancer Network. Pancreatic Adenocarcinoma 2016; version 1.
- Verma V, Li J, Lin C. Neoadjuvant therapy for pancreatic cancer: Systematic review of postoperative morbidity, mortality, and complications. Am J Clin Oncol 2016;39:302-13. [Crossref] [PubMed]
- Van Tienhoven G, Versteijne E, Suker M, et al. Preoperative chemoradiotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC-1): A randomized, controlled, multicenter phase III trial. J Clin Oncol 2018;36:abstr LBA4002.
- Neoptolemos JP, Stocken DD, Dunn JA, et al. Influence of resection margins on survival for patients with pancreatic cancer treated by adjuvant chemoradiation and/or chemotherapy in the ESPAC-1 randomized controlled trial. Ann Surg 2001;234:758-68. [Crossref] [PubMed]
- Konstantinidis IT, Warshaw AL, Allen JN, et al. Pancreatic ductal adenocarcinoma: Is there a survival difference for R1 resections versus locally advanaced unresectable tumors? What is a “true” R0 resection? Ann Surg 2013;257:731-6. [Crossref] [PubMed]
- Boone BA, Steve J, Krasinskas AM, et al. Outcomes with FOLFIRINOX for borderline resectable and locally unresectable pancreatic cancer. J Surg Oncol 2013;108:236-41. [Crossref] [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [Crossref] [PubMed]
- Tang K, Lu W, Qin W, et al. Neoadjuvant therapy for patients with borderline resectable pancreatic cancer: A systematic review and meta-analysis of response and resection percentages. Pancreatology 2016;16:28-37. [Crossref] [PubMed]
- Hasan S, Renz P, Wegner RE, et al. Microsatellite instability (MSI) as an independent predictor of pathologic complete response (PCR) in locally advanced rectal cancer. Ann Surg 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT). Ann Surg 2018;268:591-601. [Crossref] [PubMed]
- Murphy JE, Wo JY, Ryan DP, et al. Total neoadjuvant therapy with FOLFIRINOX followed by individualized chemoradiotherapy for borderline resectable pancreatic adenocarcinoma. JAMA Oncol 2018;4:963. [Crossref] [PubMed]
- Xu X, Wu Q, Wang Z, et al. Meta-analysis of FOLFIRINOX regimen as the first-line chemotherapy for locally advanced pancreatic cancer and borderline resectable pancreatic cancer. Clin Exp Med 2019;19:149-57. [Crossref] [PubMed]
- S1505: Combination Chemotherapy or Gemcitabine Hydrochloride and Paclitaxel Albumin-Stabilized Nanoparticle Formulation Before Surgery in Treating Patients With Pancreatic Cancer That Can Be Removed by Surgery. Available online: https://clinicaltrials.gov/ct2/show/NCT02562716
- Suntharalingam M, Paulus R, Edelman MJ, et al. RTOG 0229: A phase II trial of neoadjuvant therapy with concurrent chemotherapy and high-dose radiotherapy (XRT) followed by resection and consolidative therapy for LA-NSCLC. J Clin Oncol 2010;28:7024. [Crossref]
- Haque W, Verma V, Butler EB, et al. Pathologic nodal clearance and complete response following neoadjuvant chemoradiation for clinical N2 non-small cell lung cancer: Predictors and long-term outcomes. Lung Cancer 2019;130:93-100. [Crossref] [PubMed]
- Portuondo JI, Massarweh NN, Zhang Q, et al. Nodal downstaging as a treatment goal for node-positive pancreatic cancer. Surgery 2019;165:1144-50. [Crossref] [PubMed]
- Iacobuzio-Donahue CA, Fu B, Yachida S, et al. DPC4 gene status of the primary carcinoma correlates with patterns of failure in patients with pancreatic cancer. J Clin Oncol 2009;27:1806-13. [Crossref] [PubMed]
- Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Ann Diagn Pathol 2012;16:29-37. [Crossref] [PubMed]
- Barenboim A, Lahat G, Geva R, et al. Neoadjuvant FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer: An intention to treat analysis. Eur J Surg Oncol 2018;44:1619-23. [Crossref] [PubMed]
- He J, Blair AB, Groot VP, et al. Is a pathological complete response following neoadjuvant chemoradiation associated with prolonged survival in patients with pancreatic cancer? Ann Surg 2018;268:1-8. [Crossref] [PubMed]
- Verma V, Sleightholm RL, Rusthoven CG, et al. Malignant peritoneal mesothelioma: national practice patterns, outcomes, and predictors of survival. Ann Surg Oncol 2018;25:2018-26. [Crossref] [PubMed]
- Moreno AC, Haque W, Verma V, et al. Concurrent versus sequential chemoradiation therapy in completely resected pathologic N2 non-small cell lung cancer: Propensity-matched analysis of the national cancer data base. Ann Surg Oncol 2018;25:1245-53. [Crossref] [PubMed]
- Haque W, Verma V, Naik N, et al. Metaplastic breast cancer: Practice patterns, outcomes, and the role of radiotherapy. Ann Surg Oncol 2018;25:928-36. [Crossref] [PubMed]
- Verma V, Appiah AK, Lautenschlaeger T, et al. Chemoradiotherapy versus chemotherapy alone for unresected intrahepatic cholangiocarcinoma: Practice patterns and outcomes from the national cancer data base. J Gastrointest Oncol 2018;9:527-35. [Crossref] [PubMed]
- Verma V, Surkar SM, Brooks ED, et al. Chemoradiotherapy versus chemotherapy alone for unresected nonmetastatic gallbladder cancer: National practice patterns and outcomes. J Natl Compr Canc Netw 2018;16:59-65. [Crossref] [PubMed]
- Haque W, Verma V, Fakhreddine M, et al. Addition of chemotherapy to definitive radiotherapy for IB1 and IIA1 cervical cancer: Analysis of the National Cancer Data Base. Gynecol Oncol 2017;144:28-33. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Radical cystectomy versus chemoradiation for muscle-invasive bladder cancer: impact of treatment facility and sociodemographics. Anticancer Res 2017;37:5603-8. [PubMed]
- Verma V, Allen PK, Simone CB 2nd, et al. Addition of definitive radiotherapy to chemotherapy in patients with newly diagnosed metastatic nasopharyngeal cancer. J Natl Compr Canc Netw 2017;15:1383-91. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Chemotherapy Versus Chemoradiation for Node-Positive Bladder Cancer: Practice Patterns and Outcomes from the National Cancer Data Base. Bladder Cancer 2017;3:283-91. [Crossref] [PubMed]
- Verma V, Simone CB 2nd, Lin C. Human papillomavirus and nasopharyngeal cancer. Head Neck 2018;40:696-706. [Crossref] [PubMed]
- Haque W, Verma V, Butler EB, et al. Patterns of Care and Outcomes of Hypofractionated Chemoradiation Versus Conventionally Fractionated Chemoradiation for Glioblastoma in the Elderly Population. Am J Clin Oncol 2018;41:167-72. [PubMed]
- Haque W, Verma V, Butler EB, et al. Omission of radiotherapy in elderly women with early stage metaplastic breast cancer. Breast 2018;38:154-9. [Crossref] [PubMed]
- Cushman TR, Venigalla S, Brooks ED, et al. Utilization of Neoadjuvant Intensity-modulated Radiation Therapy for Rectal Cancer in the United States. Anticancer Res 2018;38:2923-7. [PubMed]
- Haque W, Verma V, Butler EB, et al. Utilization of Hysterectomy Following Chemoradiation for IB2/IIA2 Cervical Cancer in the National Cancer Data Base. Anticancer Res 2018;38:3175-9. [PubMed]
- Ryckman JM, Lin C, Simone CB 2nd, et al. Patterns of Care for Stage IA Cervical Cancer: Use of Definitive Radiation Therapy Versus Hysterectomy. Int J Gynecol Cancer 2018;28:773-81. [Crossref] [PubMed]


