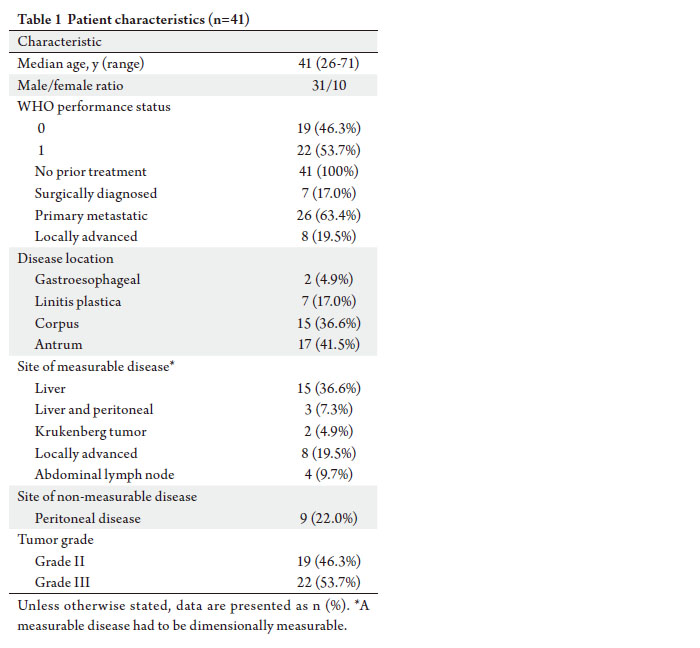
|
Discussion
Management of AGC has been evolving since the 1990’
s. Pyrhonen showed the advantage of chemotherapy
compared to best supportive care (BSC) in AGC in a small
sample size using bolus 5-FU ( 13). Findlay showed that
the administration of epirubicin, cisplatin, and continuous
infusion 5-FU (ECF) was associated with an objective
tumor response rate of 71% ( 14). These encouraging results led to a randomized trial in which ECF was compared
with FAMTX (fluorouracil-doxorubicin-methotrexate)
( 15). In that study, median survival of patients receiving
ECF (8.9 months) was also better, compared to FAMTX
(5.7 months). As a result, the benefits of infusional 5-FU
in the treatment of AGC was definitively established for
the first time in terms of clinical response and overall
survival. Folates are known to prolong the retention of the
5-f luoro-2’-deoxyuridine 5’-monophosphate (FdUMP)-
TS complex ( 16). Inhibition of TS by FdUMP is thought
to be the primary mechanism for the action of 5-FU ( 17).
A two-drug regimen consisting of cisplatin and 5-FU was
shown to decrease TS mRNA levels in adenocarcinoma
of the stomach, which explains the mechanism of action
of combination therapies ( 18). Subsequent meta-analyses
showed best results with three-drug regimens in AGC
patients ( 6). UFT is a combination (in a 1:4 M ratio) of tegafur, an oral
prodrug of 5-FU that is metabolized to 5-FU primarily in
the liver, and uracil, a natural substrate for the liver enzyme
dihydropyrimidine dehydrogenase (DPD). Compound
uracil serves as a competitive antagonist for DPD and
enhances the concentration and half-life of 5-FU ( 11, 12).
UFT is administered alone or with folinic acid (leucovorin)
tablets to increase the effect on thymidylate synthetase
(TS). Oral UFT monotherapy with leucovorin has shown
overall response rates (ORRs) of 10.5-28% and median
OS rates of 5.8-6.1 months ( 19, 20), which is similar to those reported for 5-FU single-agent continuous infusion
( 11). ORRs with two-drug regimens (UFT and cisplatin,
etoposide, or paclitaxel) were 35%-51% and average OS was
8.1-10.1 months in the treatment of AGC patients ( 21-23).
Finally, three-drug regimens with oral UFT have shown
promising results in the treatment of AGC ( 24-28). Even
complete remission of AGC has been reported using the
suppository form of UFT ( 29). UFT is absorbed readily in
the gastrointestinal system, which helps improve patient
compliance and maintain constant plasma levels of 5-FU. In
addition, catheter-related complications are avoided ( 30). Although UFT and leucovorin doses have been studied
for the last two decades, to date, an optimal administration
schedule has not been established. The goal of adding
leucovorin is to increase efficacy without additional toxicity.
Newman ( 31) and Buroker et al. ( 32) showed no survival
advantage of high-dose leucovorin but observed increased
toxicity. On the other hand, in a randomized study of colon
cancer patients, Köhne et al. found a benefit only in terms of
better progression-free survival when leucovorin was added
to 5-FU ( 33). However, this benefit was at the expense of
increased toxicity. Pazdur et al. showed that UFT with
leucovorin was equal to FUFA in colon cancer treatment,
with less toxicity in favor of UFT ( 34). No studies have
ever compared UFT versus UFT/LV treatment in gastric
and colon cancers, but colon cancer studies usually provide
guidance for approximate UFT doses. Fixed leucovorin
doses between 25 mg/m² and 90 mg/m 2 have been given to
patients, but it is primarily the UFT dose that accounts for
the overall response rate and toxicity ( 22, 27-30). Therefore, low doses of leucovorin might be recommended as opposed
to not implementing UFT at all. In this study, administration of the ECU regimen in
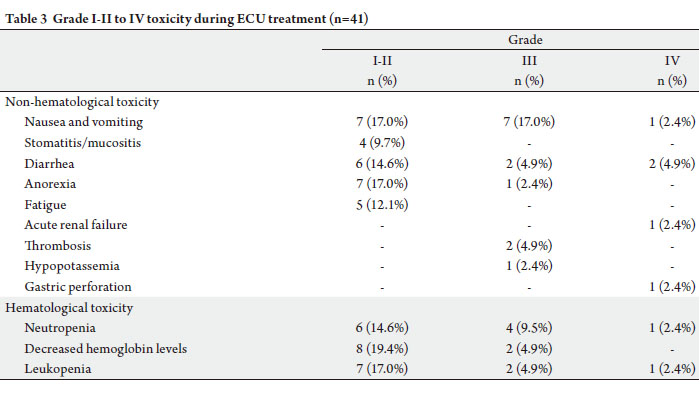
AGC patients was associated with acceptable toxicity. The
most serious toxicities observed were gastric perforation
and acute renal failure. The patient with gastric perforation
had locally advanced linitis plastica and lived for 23 months.
This is a very rare complication, with only one case reported
in the after a single cycle of UFT ( 35). Perforation may be
attributed to impaired connective tissue repair induced
by chemotherapy in the tumors ( 36) and/or it may be the
result of chemosensivity. The other serious toxicity event
was acute renal failure, which was directly related to delayed
hospitalization for grade IV diarrhea, vomiting, and nausea.
Previously, Woo reported a patient with grade IV diarrhea,
vomiting, and nausea who required a 75% reduction in
cisplatin dose ( 29), and Kim reported one case with grade
IV diarrhea that received the same three-drug UFT regimen
and required hospitalization ( 27). In this study, grade III-IV mucositis was not observed,
but grade III-IV diarrhea occurred in 4 patients (9.8%). If
UFT doses as high as 480 mg/m 2 had been used as a single
agent, more cases with grade III-IV mucositis and diarrhea
might have been observed ( 29). In a study by Kim et al.,
grade III-IV mucositis was reported in 13% of patients
receiving a UFT dose of 360 mg/m², while other studies
reported mucositis in 6% of subjects receiving 300 mg/m²
UFT in ECU regimens. The incidence of diarrhea was also
higher in the former study (10.8% vs 24-27). The incidence of grade III-IV neutropenia (11.9%) was lower in this study compared to other studies with
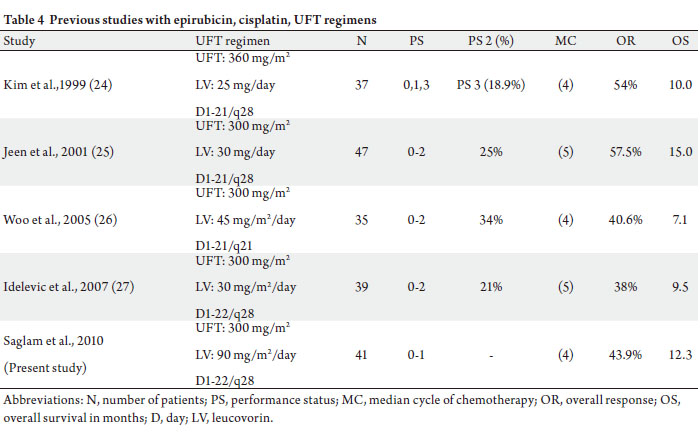
epirubicin, cisplatin, and UFT regimens ( 24-27, 29).
A 1-week drug-free interval after 3 weeks of UFT
administration, the exclusion of patients with PS 2, and
no UFT doses above 300 mg/m 2 may account for this low
incidence (Table 4). Hand-foot syndrome, neurotoxicity,
or cardiac problems were not observed in this study,
which may be attributed to the uracil component of UFT,
since it is known to prevent skin exfoliation and cardiac
events ( 37-40). Thrombosis occurred in 2 patients (4.9%).
Thrombosis is an important toxicity event during the
treatment of AGC; it occurs frequently at the initiation and
during the course of chemotherapy, resulting in poor OS
( 41). In addition to its acceptable toxicity profile and
convenience of administration on an outpatient basis,
the ECU regimen also appears to be promising in terms
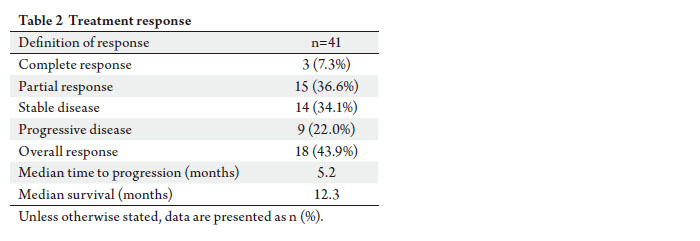
of efficacy. Overall median survival was 12.3 months
compared to 8.2 months obtained in a previous study
with the ECF regimen (epirubicin, cisplatin, infusional
5-f luorouracil) ( 14). Conversely, overall response rates
varied between 25% and 71% in studies using the ECF
regimen for AGC ( 14, 42), whereas they varied between 38%
and 54% in studies with the ECU regimen (including this
study) ( 24, 25). Therefore, the efficacy of ECU versus ECF
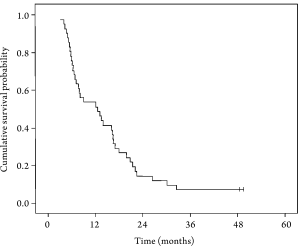
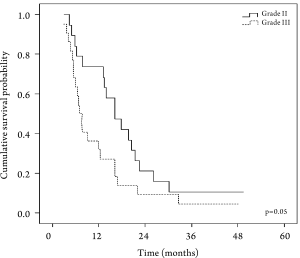
needs to be studied in larger controlled trials. One-year survival rates for Grade II and Grade III
tumors were 68.4% and 27.3%, respectively (P=0.05). The
proportion of patients with grade III tumors in this study
is close to the general profile of Turkish patients with
AGC ( 4). In future studies, the efficacy and safety of the
ECU regimen should be studied in patients with different
pathological grades. Another important factor affecting
treatment outcome is the performance status of patients
with AGC. It has a direct impact on survival, as shown in
a meta-analysis by Yoshida in AGC ( 43) . The relationship
between performance status and survival can be seen in
Table 4.
|
|
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA
Cancer J Clin 2005;55:74-108.[LinkOut]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics,
2007. CA Cancer J Clin 2007;57:43-66.[LinkOut]
- Sengelen M, Kutluk T, Fırat D. Cancer Statistics in Turkey and in the
World 1996-2003. 1st edition Turkish Association for Cancer Research
and Control, 2007. p46.
- Demir G, Zengin N, Yıldız O. Clinical profile of gastric cancer in
Turkey: Factors effecting disease free survival and relapse (Turkish
Oncology Group Study). Annals of Oncology 2006;17:Abtract :1139
and updated, unpublished data with personal communication.
- Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, et
al. Gastric cancer treated in 1991 in Japan: data analysis of nationwide
registry. Gastric Cancer 2006;9:51-66.[LinkOut]
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE.
Chemotherapy in advanced gastric cancer: a systematic review and
meta-analysis based on aggregate data. J Clin Oncol 2006;24:2903-9.[LinkOut]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M,
Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and f luorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol
2006;24:4991-7.[LinkOut]
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et
al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med 2008;358:36-46.[LinkOut]
- Moynihan T, Hansen R, Anderson T, Quebbeman E, Beatty P, Ausman
R, et al. Continuous 5-f luorouracil infusion in advanced gastric
carcinoma. Am J Clin Oncol 1988;11:461-4.[LinkOut]
- Fujii S, Nakamura Y, Takeda S, Morita K, Sato T, Marunaka T, et al.
Metabolism, antitumor activity, and acute toxicity of 5-f luoro-1,3-
bis(tetrahydro-2-furanyl)-2,4-pyrimidinedione by oral administration
to animals. Gann 1980;71:30-44.[LinkOut]
- Meropol NJ, Rustum YM, Petrelli NJ, Rodriguez-Bigas M, Frank C, Ho
DH, et al. A phase I and pharmacokinetic study of oral uracil, ftorafur,
and leucovorin in patients with advanced cancer. Cancer Chemother
Pharmacol 1996;37:581-6.[LinkOut]
- Ho DH, Pazdur R, Covington W, Brown N, Huo YY, Lassere Y, et al.
Comparison of 5-f luorouracil pharmacokinetics in patients receiving
continuous 5-f luorouracil infusion and oral uracil plus N1-(2’
-tetrahydrofuryl)-5-fluorouracil. Clin Cancer Res 1998;4:2085-8.[LinkOut]
- Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M. Randomised
comparison off luorouracil, epidoxorubicin and methotrex ate
(FEMTX) plus supportive care with supportive care alone in patients
with non-resectable gastric cancer. Br J Cancer 1995;71:587-91.[LinkOut]
- Findlay M, Cunningham D, Norman A, Mansi J, Nicolson M, Hickish
T, et al. A phase II study in advanced gastro-esophageal cancer using
epirubicin and cisplatin in combination with continuous infusion
5-fluorouracil (ECF). Ann Oncol 1994;5:609-16.[LinkOut]
- Webb A, Cunningham D, Scarffe JH, Harper P, Norman A, Joffe JK, et
al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil
versus f luorouracil, doxorubicin, and methotrexate in advanced
esophagogastric cancer. J Clin Oncol 1997;15:261-7.[LinkOut]
- Okabe H, Toko T, Saito H, Nakano K, Fujioka A, Yuasa C, et al. Augmentation of the chemotherapeutic effectiveness of UFT, a
combination of tegafur [1-(2-tetrahydrofuryl)-5-f luorouracil] with
uracil, by oral l-leucovorin. Anticancer Res 1997;17:157-64.[LinkOut]
- Copur S, Aiba K, Drake JC, Allegra CJ, Chu E. Thymidylate synthase
gene amplification in human colon cancer cell lines resistant to
5-fluorouracil. Biochem Pharmacol 1995;49:1419-26.[LinkOut]
- Lenz HJ, Leichman CG, Danenberg KD, Danenberg PV, Groshen S,
Cohen H, et al. Thymidylate synthase mRNA level in adenocarcinoma
of the stomach: a predictor for primary tumor response and overall
survival. J Clin Oncol 1996;14:176-82.[LinkOut]
- Kim YH, Cheong SK, Lee JD, Park JS, Shin SW, Kim JS. Phase II trial
of Oral UFT and leucovorin in advanced gastric carcinoma. Am J Clin
Oncol 1996;19:212-6.[LinkOut]
- Ravaud A, Borner M, Schellens JH, Geoffrois L, Schoffski BP, Kroon K,
et al. UFT and leucovorin in first-line chemotherapy for patients with
metastatic gastric cancer. An Early Clinical Studies Group (ECSG)/
European Organization for Research Treatment of Cancer (EORTC)
phase II trial. Eur J Cancer 2001;37:1642-7.[LinkOut]
- Suga S, Iwase H, Shimada M, Nishio Y, Ichihara T, Ichihara S, et al.
Neoadjuvant chemotherapy in scirrhous cancer of the stomach using
uracil and tegafur and cisplatin. Intern Med 1996;35:930-6.[LinkOut]
- Sato A, Kurihara M, Koizumi W. A phase II study of UFT plus cisplatin
(UFTP) therapy in patients with advanced gastric cancer. Proc Am Soc
Clin Oncol 2000;19:Abstr 1087.
- Feliu J, Gonzalez Baron M, Garcia-Giron C, Espinosa E, Garcia-Alfonso
P, Belon J, et al. Treatment of patients with advanced gastric carcinoma
with the combination of etoposide plus oral tegafur modulated by uracil
and leucovorin. A phase II study of the ONCOPAZ Cooperative Group.
Cancer 1996;78:211-6.[LinkOut]
- Kim YH, Kim BS, Seo JH, Choi CW, Kim JS, Chun HJ, et al. Epirubicin,
cisplatin, oral UFT, and calcium folinate in advanced gastric carcinoma.
Oncology (Williston Park) 1999;13:64-8.[LinkOut]
- Jeen YT, Yoon SY, Shin SW, Kim BS, Mok YJ, Kim CS, et al. Phase II
trial of epirubicin, cisplatin, oral uracil and tegafur, and leucovorin in
patients with advanced gastric carcinoma. Cancer 2001;91:2288-93.[LinkOut]
- Woo IS, Moon DH, Shim BY, Lee MA, Byun JH, Kim KW, et al. A phase
II study of epirubicin, cisplatin and uracil-tegafur for advanced gastric
carcinoma. Jpn J Clin Oncol 2005;35:13-7.[LinkOut]
- Idelevich E, Karminsky N, Dinerman M, Katsenelson RL, Zvi NB,
Baruch NB, et al. Phase II study of cisplatin, epirubicin, UFT, and
leucovorin (PELUF) as first-line chemotherapy in metastatic gastric
cancer. Acta Oncol 2007;46:324-9.[LinkOut]
- Aykan NF, Idelevich E. The role of UFT in advanced gastric cancer. Ann
Oncol 2008;19:1045-52.[LinkOut]
- Hirose S, Hirahara K, Aoki S, Nakagawa H, Nishimura K, Hori M, et al.
A case of complete regression of gastric carcinoma and liver metastases
by treatment with tegafur. Gan To Kagaku Ryoho 1985;12:957-9.[LinkOut]
- Vescia S, Baumgartner AK, Jacobs VR, Kiechle-Bahat M, Rody A, Loibl
S, et al. Management of venous port systems in oncology: a review of
current evidence. Ann Oncol 2008;19:9-15.[LinkOut]
- Newman EM, Akman SA, Harrison JS, Leong LA, Margolin KA, Morgan RJ, et al. Pharmacokinetics and toxicity of continuous infusion
(6S)-folinic acid and bolus 5-f luorouracil in patients with advanced
cancer. Cancer Res 1992;52:2408-12.[LinkOut]
- Buroker TR, O’Connell MJ, Wieand HS, Krook JE, Gerstner JB,
Mailliard JA, et al. Randomized comparison of two schedules of
f luorouracil and leucovorin in the treatment of advanced colorectal
cancer. J Clin Oncol 1994;12:14-20.[LinkOut]
- Kohne CH, Wils J, Lorenz M, Schoffski P, Voigtmann R, Bokemeyer
C, et al. Randomized phase III study of high-dose f luorouracil given
as a weekly 24-hour infusion with or without leucovorin versus bolus
f luorouracil plus leucovorin in advanced colorectal cancer: European
organization of Research and Treatment of Cancer Gastrointestinal
Group Study 40952. J Clin Oncol 2003;21:3721-8.[LinkOut]
- Pazdur R, Doulliard JY, Skillings J. Multicenter phase III study of
5-fluorouracil (5-FU) or UFT in combination with leucovorin (LV) in
patients with metastatic colorectal cancer (Meeting abstract). Proc Am
Soc Clin Oncol 1999:Abst 1009.
- Imai H, Obana N, Iwabuchi T, Satou Y, Ooyauchi M, Igarashi T, et al.
Long survival of advanced gastric cancer patient after total gastrectomy
and postoperative treatment with S-1 despite S-1+CDDP+CPT-11
causing perforation. Gan To Kagaku Ryoho 2008;35:1185-8.[LinkOut]
- Bozdag AD, Peker Y, Derici H, Gurkok C, Ozgonul M. The effect of
preoperative 5-fluorouracil on colonic healing: an experimental study.
Hepatogastroenterology 2001;48:1631-4.[LinkOut]
- Yamamoto J, Haruno A, Yoshimura Y, Unemi N, Kunimune Y,
Yamashita K, et al. Effect of coadministration of uracil on the toxicity of
tegafur. J Pharm Sci 1984;73:212-4.[LinkOut]
- Houghton JA, Houghton PJ, Wooten RS. Mechanism of induction of
gastrointestinal toxicity in the mouse by 5-fluorouracil, 5-fluorouridine,
and 5-fluoro-2’-deoxyuridine. Cancer Res 1979;39:2406-13.[LinkOut]
- Bagrij T, Kralovanszky J, Gyergyay F, Kiss E, Peters GJ. Influence of
uridine treatment in mice on the protection of gastrointestinal toxicity
caused by 5-fluorouracil. Anticancer Res 1993;13:789-93.[LinkOut]
- Codacci-Pisanelli G, Noordhuis P, van der Wilt CL, Peters GJ. Selective
protection by uridine of growth inhibition by 5-f luorouracil (5FU)
mediated by 5FU incorporation into RNA, but not the thymidylate
synthase mediated growth inhibition by 5FU-leucovorin. Nucleosides
Nucleotides Nucleic Acids 2008;27:733-9.[LinkOut]
- Tetzlaff ED, Correa AM, Baker J, Ensor J, Ajani JA. The impact on
survival of thromboembolic phenomena occurring before and during
protocol chemotherapy in patients with advanced gastroesophageal
adenocarcinoma. Cancer 2007;109:1989-95.[LinkOut]
- Roth AD, Fazio N, Stupp R, Falk S, Bernhard J, Saletti P, et al.
Docetaxel, cisplatin, and fluorouracil; docetaxel and cisplatin; and
epirubicin, cisplatin, and f luorouracil as systemic treatment for
advanced gastric carcinoma: a randomized phase II trial of the Swiss
Group for Clinical Cancer Research. J Clin Oncol 2007;25:3217-23.[LinkOut]
- Yoshida M, Ohtsu A, Boku N, Miyata Y, Shirao K, Shimada Y, et al.
Long-term survival and prognostic factors in patients with metastatic
gastric cancers treated with chemotherapy in the Japan Clinical
Oncology Group (JCOG) study. Jpn J Clin Oncol 2004;34:654-9.[LinkOut]
Cite this article as:
Saglam S, Aykan N, Sakar B, Gulluoglu M, Balik E, Karanlik H. A pilot study evaluating the safety and toxicity of epirubicin,
cisplatin, and UFT (ECU regimen) in advanced gastric
carcinoma. J Gastrointest Oncol. 2011;2(1):19-26. DOI:10.3978/j.issn.2078-6891.2010.030
|