
A case of class 3 MEK1 mutated metastatic colorectal cancer with a non-durable tumor marker response to MEK and ERK inhibitors
Case presentation
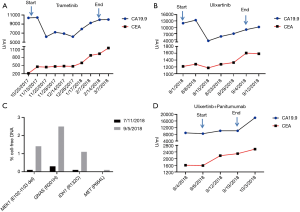
A 52-year-old female presented with obstructive symptoms and abdominal pain, which led to a diagnosis of descending colon adenocarcinoma with metastatic disease to the lungs, liver and retroperitoneal lymph nodes. Left hemicolectomy revealed a poorly differentiated adenocarcinoma with signet-ring and mucinous features. She received first-line chemotherapy with FOLFOX for 5 cycles with stable disease (SD) as best response. In the interim, next generation sequencing (NGS) of her primary tumor by FoundationOne® (Gene List: https://www.foundationmedicine.com/genomic-testing/foundation-one-cdx, Foundation Medicine, Inc., Cambridge, MA, USA) revealed a microsatellite stable, KRAS and NRAS wild type, BRAF wild type, IDH1 R132c [mutation allele frequencies (MAF) 22.18%] mutation, FANCG loss exons 5–14, GNAS R201H (MAF, 38.69%), and aMEK1 (E102-I103del, MAF, 21.87%) mutation. Given the lack of RAS and BRAF mutations and the concerns about prior anastomotic micro-perforation, panitumumab was added to her treatment regimen. She progressed after an additional 4 cycles of FOLFOX plus panitumumab chemotherapy. Given her refractoriness to first-line chemotherapy and emerging case reports of MEK1 mutated tumors responding to MEK inhibitor, she was treated with trametinib (1-3). She experienced a short-lived decline in CA19.9 (Figure 1A). Unfortunately, she had progressive disease (PD) on CT scan after 3 months of trametinib treatment. She was then treated with FOLFIRI plus bevacizumab with SD as best response, followed by PD at after 5 months of treatment. Given emerging pre-clinical data characterizing MEK1 (E102_I103) mutation as a class 3 MEK1 mutation with relative resistance to MEK inhibitors and with sensitivity to ERK inhibitors, we treated our patient on a single-patient Investigational New Drug (IND)-exempt clinical trial of the ERK inhibitor, ulixertinib (BVD-523) (4-6). The study was approved by City of Hope Investigational Review Board (IRB #18278). Ulixertinib was administrated orally twice-daily at the previously recommended phase II dose of 600 mg PO BID (6). Two weeks after initiation of ulixertinib, the patient experienced a more robust, but short-lived, decline in CA19.9 (Figure 1B). The treatment was well-tolerated with mild to moderate nausea, fatigue, and dry skin (grade 1). Unfortunately, she experienced a subsequent surge in tumor markers and radiographic progression following 6 weeks of treatment. Meanwhile, digital NGS of cell-free DNA (cfDNA) carried out by Guardant360 cfDNA assay (Gene List: http://www.guardant360.com/, Guardant Health, Redwood City, CA, USA) revealed an increased proportion of MEK1 cfDNA (0.095589–1.41%), GNAS R201H cfDNA (0.3–2.5%), IDH1 R132C cfDNA (0.1–1.1%), and MET (non-detectable–0.1%), with the detection of a synonymous PIK3CA mutation, which suggests increased tumor load and tumor evolution but without clear explanation of the resistance mechanisms (Figure 1C). Given the concern that the rapid acquired resistance was related to compensatory EGFR phosphorylation, we amended her clinical trial to allow for the combination of ulixertinib plus panitumumab. Following IRB approval and after obtaining patient consent, ulixertinib was continued at 600 mg PO BID in combination with panitumumab 6 mg/kg given intravenously every 2 weeks. Unfortunately, this treatment was poorly tolerated with refractory nausea and vomiting requiring admission following 2 weeks of treatment. Imaging studies at the time of admission did not show any obstructive findings and her disease burden was noted to be unchanged in this short period of treatment. Given the intolerance to protocol treatment and lack of disease regression or tumor marker decline (Figure 1D) with ulixertinib plus panitumumab, the patient was taken off study. She was subsequently treated with trifluridine/tipiracil with rapid disease progression and death.
Discussion
Mutations in MEK1 occur in approximately 1–2% of colorectal cancer patients, and have been characterized as oncogenic (7,8). Preclinical studies confirm that MEK1 activating mutations are sufficient to transform intestinal epithelial cells and facilitate the formation of high-grade adenocarcinoma (9). MEK1 mutations have been classified into 3 classes. Class 1 MEK1 mutations are RAF-dependent and are the least activating. Class 2 MEK1 mutations are activating in nature but can be upregulated further by upstream RAF. Class 3 MEK1 mutations (∆L98-I103, ∆I99-K104, ∆E102-I103, ∆I103-K104) lead to auto-phosphorylation of MEK which is independent of RAF and are associated with the highest level of downstream ERK phosphorylation (4). Class 3 MEK1 mutations are mutually exclusive with other mutations that activate MAPK signaling, and are therefore considered driver mutations. In addition, class 3 mutations promote tumor formation in mice more efficiently than class 1 and class 2 mutations, suggesting that this class is the most oncogenic amongst MEK1 mutations (4). Among solid tumors, MEK1 mutations have been best characterized in non-small cell lung cancer (NSCLC), where class 2 mutations (K57N and Q56P) were the most common and were associated with a worse outcome in the setting of metastatic disease (10).
Gao et al. have previously demonstrated the relative resistance of class 3 MEK1 mutations to MEK inhibition (4). Prior to Gao’s report, we treated our patient with trametinib, and her disease indeed progressed after 3 months of trametinib. However, trametinib was associated with complete remission in a case of class 3 MEK1 mutation (∆E102-I103) Langerhans cell histiocytosis (LCH) and with PD in another class 3 MEK1 mutation (∆L98- K104) LCH patient (3,11). Complete responses to MEK inhibitors have also been described in two cases with histiocytic sarcoma and serous ovarian cancer, both harboring class 2 MEK1 mutations (1,2).
Given this patient’s resistance to multiple lines of chemotherapy and to trametinib, and in light of pre-clinical and clinical work suggesting benefit from ERK inhibition (4,6). We treated our patient with ulixertinib alone and added panitumumab to ulixertinib at the time of progression, on a single patient IND-exempt clinical trial. In line with our expectations, treatment with ulixertinib resulted a steeper decline in CA19.9 than with trametinib, but this response was short lived and disease progression was recorded four weeks after starting ulixertinib. CEA and CA19.9 tumor markers are often elevated in the setting of metastatic colorectal cancer. Comparative studies between CEA and imaging studies show a very strong correlation between CEA response and imaging response. Combining with the initial decline of CEA and subsequent rise in those markers, our results suggest an initial response with rapid onset of acquired resistance to ERK inhibition (12). Circulating cfDNA assays showed an emergent PIK3CA mutation at the time of resistance. This specific mutation causes a synonymous alteration which is not likely the underlying mechanism of resistance. Adding panitumumab to her treatment did not reverse this resistance, which suggests that the mechanism of resistance is not likely limited to compensatory EGFR phosphorylation. Unfortunately, no serial tumor biopsies were obtained during treatment, making the mechanistic evaluation of resistance more challenging.
Disparity in sensitivity to BRAF inhibitors between melanoma and colorectal cancer suggests primary tumor heterogeneity despite harboring identical BRAF mutations (13,14). This case highlights similar heterogeneity in response to MEK or ERK inhibitors within MEK1 mutated tumors and highlights the need of better pre-clinical models to guide drug development in this rare molecularly defined subgroup. While similar mutations to our case have responded to MEK inhibition in the setting of LCH, no clinical response was noted in our case with either MEK inhibitors or ERK inhibitors +/− anti-EGFR. The initial response in tumor markers followed by rapid disease progression suggests rapid compensatory mechanisms that bypass in MEK and ERK inhibition in CRC. Future studies investigating ERK or MEK inhibitors in tumors harboring MEK1 mutations should include robust correlative studies and PDX modeling to better delineate the various mechanisms of resistance and to develop better rational combinations for this patient population.
Acknowledgments
None.
Footnote
Conflicts of Interest: M Fakih: Amgen [Honoraria, Advisory/Consultancy, Speaker Bureau/Expert Testimony, Research Grant (Institution)], Array (Advisory/Consultancy), Bayer (Advisory/Consultancy), Astra Zeneca [Research Grant (Institution)], Novartis [Research Grant (Institution)]. C Wang, J Sandhu have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
- Gounder MM, Solit DB, Tap WD. Trametinib in Histiocytic Sarcoma with an Activating MAP2K1 (MEK1) Mutation. N Engl J Med 2018;378:1945-7. [Crossref] [PubMed]
- Grisham RN, Sylvester BE, Won H, et al. Extreme Outlier Analysis Identifies Occult Mitogen-Activated Protein Kinase Pathway Mutations in Patients With Low-Grade Serous Ovarian Cancer. J Clin Oncol 2015;33:4099-105. [Crossref] [PubMed]
- Papapanagiotou M, Griewank KG, Hillen U, et al. Trametinib-Induced Remission of an MEK1-Mutated Langerhans Cell Histiocytosis. JCO Precis Oncol 2017;1:1-5.
- Gao Y, Chang MT, McKay D, et al. Allele-Specific Mechanisms of Activation of MEK1 Mutants Determine Their Properties. Cancer Discov 2018;8:648-61. [Crossref] [PubMed]
- Hatzivassiliou G, Liu B, O'Brien C, et al. ERK inhibition overcomes acquired resistance to MEK inhibitors. Mol Cancer Ther 2012;11:1143-54. [Crossref] [PubMed]
- Sullivan RJ, Infante JR, Janku F, et al. First-in-Class ERK1/2 Inhibitor Ulixertinib (BVD-523) in Patients with MAPK Mutant Advanced Solid Tumors: Results of a Phase I Dose-Escalation and Expansion Study. Cancer Discov 2018;8:184-95. [Crossref] [PubMed]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330-7. [Crossref] [PubMed]
- Choi YL, Soda M, Ueno T, et al. Oncogenic MAP2K1 mutations in human epithelial tumors. Carcinogenesis 2012;33:956-61. [Crossref] [PubMed]
- Voisin L, Julien C, Duhamel S, et al. Activation of MEK1 or MEK2 isoform is sufficient to fully transform intestinal epithelial cells and induce the formation of metastatic tumors. BMC Cancer 2008;8:337. [Crossref] [PubMed]
- Arcila ME, Drilon A, Sylvester BE, et al. MAP2K1 (MEK1) Mutations Define a Distinct Subset of Lung Adenocarcinoma Associated with Smoking. Clin Cancer Res 2015;21:1935-43. [Crossref] [PubMed]
- Azorsa DO, Lee DW, Wai DH, et al. Clinical resistance associated with a novel MAP2K1 mutation in a patient with Langerhans cell histiocytosis. Pediatr Blood Cancer 2018;65:e27237. [Crossref] [PubMed]
- Iwanicki-Caron I, Di Fiore F, Roque I, et al. Usefulness of the serum carcinoembryonic antigen kinetic for chemotherapy monitoring in patients with unresectable metastasis of colorectal cancer. J Clin Oncol 2008;26:3681-6. [Crossref] [PubMed]
- Corcoran RB, Atreya CE, Falchook GS, et al. Combined BRAF and MEK Inhibition With Dabrafenib and Trametinib in BRAF V600–Mutant Colorectal Cancer. J Clin Oncol 2015;33:4023-31. [Crossref] [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [Crossref] [PubMed]


