
Malignant gastrointestinal neuroectodermal tumor, presenting as a second malignancy after gastric adenocarcinoma: a case report and literature review
Background
Malignant gastrointestinal neuroectodermal tumor (GNET), previously known as clear cell sarcoma-like tumor of the gastrointestinal tract (CCSLT-GT), is a rare mesenchymal tumor mainly occurring in gastrointestinal tract (1). In 1985, a tumor in jejunum characterized as “malignant neuroendocrine tumor with osteoclast-like giant cell” was firstly reported (2). Then in 2003, six cases of clear cell sarcomas in gastrointestinal tract were collected and its common features of pathological entity were concluded (3). Microscopically, it is characterized by epithelioid cells with clear or eosinophilic cytoplasm that grow in nest and presence of osteoclast-like giant cells. Immunohistochemically, it usually shows diffuse and strong reactivity for S100 and is negative for cytokeratin, HMB-45, A103. Recently, the translocation of EWSR1 has been documented, and identification of this genetic derangement is useful for diagnosis. Biologically, GNET is extremely malignant and prone to local recurrence and metastasis (4). Nevertheless, its etiology is still not clear. Here we described a case of a 30-year-old woman, who received chemotherapy for gastric adenocarcinoma and subsequently developed a new neoplasm of GNET in the small bowel wall.
Case presentation
In 2004, a 19-year-old woman was diagnosed as poorly differentiated adenocarcinoma of the stomach. The carcinoma invaded into the main muscular layer and spread to four lymph nodes, which was classified as stage IIB. Then a total gastrectomy and five cycles of EOF (oxaliplatin + epirubicin + calcium folinate + 5-FU) chemotherapy was conducted. There was no recurrence or metastasis in patient follow-up until 2015 when she presented symptoms of abdominal distension and vomiting. Computer tomography (CT) scan of abdomen and pelvis showed an obstruction in the small intestine. Emergent exploratory surgery revealed a mass located in the middle of intussusception of the ileum, and an excision of the ileum mass was performed. It was thought to be a metastasis of previous gastric adenocarcinoma clinically.
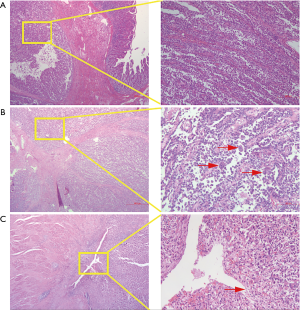
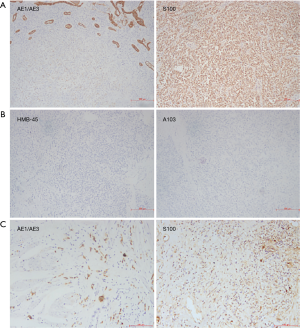
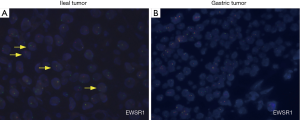
Macroscopically, resected ileum showed a 3×2.7×2 cm3 mass. The cutting surface was grey-white. Microscopic findings revealed that the tumor was located under the ileum mucosa, arranged in a solid and pseudopapillary pattern (Figure 1A). Characteristically, the cells were polygonal in shape, contained clear to eosinophilic cytoplasm and oval nuclei with a single small nucleolus. Scattered osteoclast-like multinucleated giant cells were also identified (Figure 1B). Mitotic Figures were found in some fields, but necrosis and ulcer were not extensive. To carefully exclude that the neoplasm was not a metastatic lesion of gastric tumor, we reviewed the slides of the past gastric tumor. The histological and immunohistological findings were quite different. Gastric tumor cells were poorly cohesive with occasional signet-ring cells (Figure 1C), and the cells were positive for AE1/AE3 cytokeratin, confirming its epithelial origin. The GNET cells were strongly and diffusely positive for S100 staining, but were negative for AE1/AE3 cytokeratin, A103 and HMB-45 (Figure 2). Thus, we considered ileum lesion was a second new neoplasm of different origin in digestive tract compared with previous gastric tumor. To further confirm the results, we conducted fluorescence in situ hybridization (FISH) analysis for EWSR1 rearrangement. Fifty percent of the cells showed a split signal of EWSR1 in ileum tumor (Figure 3A), while in stomach tumor rearrangement of EWSR1 was not detected (Figure 3B).
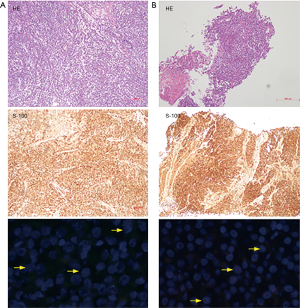
One month after surgical resection, the patient received chemotherapy. During the clinical follow-up, the patient kept disease free for 14 mouths. However, she presented a mesentery mass of small intestine in September 2016. Then she received a surgical removal of the mesentery mass. It turned out to be GNET metastasis through pathological examination (Figure 4A). After she recovered from surgery, unfortunately, she was found to have mesenteric and lung lesions by the CT scan in January 2018. By conducting percutaneous lung biopsy under the CT, the lesion was confirmed to be a GNET metastasis (Figure 4B). The patient was then switched to take sunitinib and still alive till January 2019.
Discussion
Malignant GNET is a rare malignant neoplasm arising in the intestinal tract, stomach, or colon, and occurs predominantly in young adults. Patients usually develop anemia and abdominal pain (5,6). CT scan of the abdomen and pelvis helps to detect lesion. GNET is usually associated with high rate of local recurrence, metastasis and its average survival time is 18.5 months (1,3). Until now, the etiology of the neoplasm is still unclear and its tumorigenesis is obscure at the initial stages of development.
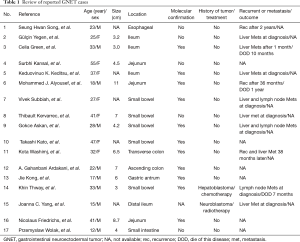
We reviewed published case reports of GNET, most of which have no history of previous malignancy of any different kind (Table 1). Only two of them were present as a second primary GNET after previously known malignancy. Both patients had been treated with surgery plus low-dose radiotherapy or surgery plus chemotherapy for the previously neoplasm (7-9). One of these patients was diagnosed as hepatoblastoma at age of 13, then he was treated with surgery and followed by six cycles of PLADO regimen chemotherapy (cisplatin and doxorubicin), who eventually developed GNET 20 years later (7). The same as above, our patient was diagnosed as poorly differentiated adenocarcinoma of stomach 14 years ago, and then received total gastrectomy and five cycles of EOF chemotherapy (oxaliplatin 150 mg 1d + epirubicin 60 mg day 1 + calcium folinate 0.1 g days 1–5 + 5-FU 0.5 g days 1–5). We realized that these two patients underwent similar chemotherapy agents when they were young and developed the same disease eventually, which suggested that chemotherapy might play a role in the tumorigenesis of GNET.
Full table
Platinum drugs were conventional chemotherapy drugs for many different solid tumors, including gastric cancer (10). While doxorubicin was a routine chemotherapy drug in breast cancer, which was rarely administered in gastric cancer in recent years. Actually, combined chemotherapy with platinum and epirubicin was a routine treatment strategy in gastric cancer in early years (11,12). Interestingly, the risk of gastric adenocarcinoma increased after chemotherapy of diffuse large B cell lymphoma (13,14), and yet secondary malignant tumors after gastric adenocarcinoma chemotherapy were rarely reported. Moreover, there were rare reports showing that platinum-based drugs and doxorubicin, alone or combination, would induce a new neoplasm.
Generally, advanced poorly differentiated gastric adenocarcinoma progresses quickly, and five-years overall survival rate is 31%. However, the patient in our case has lived a long life after gastric surgery and chemotherapy. We hypothesized that there might be some unknown germline mutation which played an important role in the natural history of gastric carcinoma in this case, and eventually contributed to the small bowel lesion. Furthermore, germline mutation and prolonged survival made the carcinogenicity of chemotherapy more obvious. Thus, more work should be done to demonstrate if the combination of platinum-based drugs and doxorubicin could contribute to increased possibility of EWSR1 rearrangement, which might cause the onset of GNET.
Acknowledgments
Funding: This report was supported by National Science Foundation of China (No. 81702875).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this Case report and any accompanying images.
References
- Stockman DL, Miettinen M, Suster S, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol 2012;36:857-68. [Crossref] [PubMed]
- Alpers CE, Beckstead JH. Malignant neuroendocrine tumor of the jejunum with osteoclast-like giant cells. Enzyme histochemistry distinguishes tumor cells from giant cells. Am J Surg Pathol 1985;9:57-64. [Crossref] [PubMed]
- Zambrano E, Reyes-Mugica M, Franchi A, et al. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol 2003;11:75-81. [Crossref] [PubMed]
- Insabato L, Guadagno E, Natella V, et al. An unusual association of malignant gastrointestinal neuroectodermal tumor (clear cell sarcoma-like) and Ewing sarcoma. Pathol Res Pract 2015;211:688-92. [Crossref] [PubMed]
- Kong J, Li N, Wu S, Guo X, et al. Malignant gastrointestinal neuroectodermal tumor: A case report and review of the literature. Oncol Lett. 2014;8:2687-90. [Crossref] [PubMed]
- Alyousef MJ, Alratroot JA, ElSharkawy T, et al. Malignant gastrointestinal neuroectodermal tumor: a case report and review of the literature. Diagn Pathol 2017;12:29. [Crossref] [PubMed]
- Thway K, Judson I, Fisher C. Clear cell sarcoma-like tumor of the gastrointestinal tract, presenting as a second malignancy after childhood hepatoblastoma. Case Rep Med 2014;2014:984369. [Crossref] [PubMed]
- Yang JC, Chou AJ, Oeffinger KC, et al. Clear cell sarcoma of the gastrointestinal tract after very low-dose therapeutic radiation therapy: a case report. J Pediatr Surg 2012;47:1943-5. [Crossref] [PubMed]
- Balkaransingh P, Saad SA, Govil SC, et al. Clear cell sarcoma of the gastrointestinal tract presenting as a second malignant neoplasm following neuroblastoma in infancy. Pediatr Blood Cancer 2012;58:481-2. [Crossref] [PubMed]
- Gridelli C, Morabito A, Cavanna L, et al. Cisplatin-Based First-Line Treatment of Elderly Patients With Advanced Non-Small-Cell Lung Cancer: Joint Analysis of MILES-3 and MILES-4 Phase III Trials. J Clin Oncol 2018;36:2585-92. [Crossref] [PubMed]
- Allum WH. Combination chemotherapy with epirubicin, cisplatin and 5-fluorouracil for the palliation of advanced gastric and oesophageal adenocarcinoma. The MRC Gastric Cancer Working Party and the British Stomach Cancer Group. Br J Surg 1995;82:565. [Crossref] [PubMed]
- Aitini E, Rabbi C, Mambrini A, et al. Epirubicin, cisplatin and continuous infusion 5-fluorouracil (ECF) in locally advanced or metastatic gastric cancer: a single institution experience. Tumori 2001;87:20-4. [Crossref] [PubMed]
- Inaba K, Kushima R, Murakami N, et al. Increased risk of gastric adenocarcinoma after treatment of primary gastric diffuse large B-cell lymphoma. BMC Cancer 2013;13:499. [Crossref] [PubMed]
- Sakr R, Massoud M, Aftimos G, et al. Gastric Adenocarcinoma Secondary to Primary Gastric Diffuse Large B-cell Lymphoma. J Gastric Cancer 2017;17:180-5. [Crossref] [PubMed]


