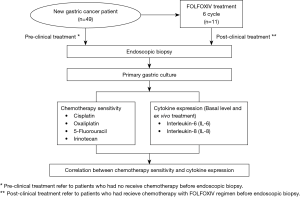
Interleukin-8 associated with chemosensitivity and poor chemotherapeutic response to gastric cancer
Introduction
Gastric cancer (GC) is a challenging cancer to treat with the 5-year survival rate recorded at only 10% (1). This is due to its high rate of therapy-resistant local recurrences after surgical resection or chemoembolization. Among the four Thai regions, the northern region revealed the highest incidence rates of GC (6.45 for men and 4.35 for women), whereas the southern region revealed the lowest rates (1.9 for men and 1.4 for women) (2). However, there has been a limited amount of studies on GC cases in Thailand. This study will provide important information on the incidences of GC sub-types and the association of IL-8 expression with regard to chemosensitivity, as well as to offer a prediction of poor chemotherapeutic responses among GC patients in endemic areas of Thailand.
Currently, there is not a standard regimen worldwide for chemotherapy treatment among GC patients. A number of chemotherapeutic drugs have been used to treat GC including 5-fluorouracil (5-FU), capecitabine, oxaliplatin, cisplatin, docetaxel, epirubicin, irinotecan, oxaliplatin and paclitaxel (3). Our center, Maharaj Nakorn Chiang Mai Hospital, has treated the majority of GC patients in the northern region. Many of these patients have been transferred from community hospitals located in 17 provinces across the northern region of Thailand. GC patients are typically treated with oxaliplatin in combination with 5-FU/leucovorin (FOLFOXIV regimen) as the first line of drug treatment, and FOLFIRI (irinotecan + 5-FU/leucovorin) as a second line of drug treatment. However, some patients have developed chemotherapy resistance, which has resulted in a lowering of their overall survival rates. Therefore, it is important to predict the occurrence of chemosensitivity using ex vivo primary gastric cultures obtained from endoscopic biopsies for optimal drug selection in clinical treatments. Moreover, identifying the predictive biomarkers for treatment would likely improve the clinical outcomes for GC patients.
Cytokines play a functional role in the natural history of various cancer diseases (4-6). Elevated levels of inflammatory cytokines, such as interleukin-6 (IL-6) and interleukin-8 (IL-8), are known to be associated with cancer cell proliferation, angiogenesis and metastasis (7-10). Recently, there have been reports that levels of IL-6 in the blood of GC patients were higher than in the healthy control group and the levels of IL-8 in the blood correlate with late stage of GC (11). Our previous report demonstrated that high levels of IL-8 mRNA expression were detected in more than 80% of Thai GC patients (12). The association between overexpression of IL-8 and the response to chemotherapy and the prognosis has been reported for head and neck cancer patients, hepatocellular carcinoma cases and in GC cell lines (13,14).
However, no recent reports have been published that acknowledge a correlation of IL-6 and IL-8 levels and ex vivo chemosensitivity to drug treatment in the primary gastric cultures obtained from patients. Therefore, we hypothesize that recording the cytokine IL-6 and IL-8 expression levels can be useful in terms of assessing the diagnostic and prognostic risks and the predictive markers for GC treatment.
In the current research study, a primary gastric culture obtained from a patient’s endoscopic biopsy was established. Preliminarily, we investigated whether the ex vivo chemosensitivity to drugs may be correlated with the protein expressions of IL-6 and IL-8, along with the linkage to clinical responses. Our data suggests that the primary gastric culture could serve as a valuable tool for chemotherapy screening, and that the repeated usage of platinum drugs may cause drug resistance via upregulation of IL-8 levels.
Methods
Materials and drug preparation
Dulbecco’s modified Eagle’s medium (DMEM), penicillin-streptomycin, and trypsin-ethylenediaminetetra-acetic acid (EDTA) were purchased from GIBCO-BRL (Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from Hyclone (Logan, UT, USA). Cisplatin, oxaliplatin, 5-FU and irinotecan were purchased from Sigma-Aldrich (St Louis, MO, USA). Cisplatin stock solutions were prepared at concentrations of 5 mM. 5-FU, while oxaliplatin and irinotecan stock solutions were prepared at a concentration of 10 mM. For each experiment, all drugs were freshly prepared and diluted with the appropriate solvent to achieve the final treatment concentrations.
Clinical sample collection and data recording
The study included 49 patients with GC who were admitted to Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand, and 38 patients underwent primary surgical resection between June 2014 and December 2016. There are 38 patients who underwent resection with adjuvant chemotherapy and 11 patients this group are received neoadjuvant therapy. The researchers of this study obtained the written informed consent of all the participants. All 49 patients had not undergone any chemotherapy treatments before the endoscopic biopsy. Among them, 11 patients had an endoscopic biopsy again after receiving chemotherapy with the FOLFOXIV regimen. All of patients were observed until the completion of the study or their deaths. All specimens were processed within a day of sample collection. The clinical features of all patients, including age at diagnosis, gender, site of disease, surgery type and histological type were obtained from clinical and pathological reports. All patients were diagnosed according to the criteria of the World Health Organization (WHO) and Lauren Classification.
Ethical consideration
This study was approved by the Chiang Mai University Ethics Committee for Human Research. The ethics number and the study code number are 408/2015 and SUR-2558-03015, respectively with written informed consent being obtained prior to use of the endoscopic biopsy samples. All human studies have been reviewed by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in an appropriate version of the Declaration of Helsinki. The researchers of this study are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Primary gastric culture from endoscopic biopsy
The endoscopic biopsy tissue samples at the tumor site (a size of 2 mm3) was done by an expert surgeon at the Department of Surgery, Maharaj Nakorn Chiang Mai Hospital, Chiang Mai, Thailand with written informed consent of all participants. Tissue specimens were kept in culture medium and then immediately transfer to cell culture laboratory. Each of the diagnoses was histopathological confirmed by a senior pathologist. The primary culture of GC was performed according to previously described protocol (15). Briefly, the tissue samples were added into the enzyme mixture (collagenase A 0.15 U/mL, dispase 2.4 U/mL and FBS diluted in in-complete medium) and mixed every 10 minutes for 1 hour. The tissue was then pelleted after centrifugation at 4,500 g for 5 minutes. The cell pellets were washed twice with 10 mL DMEM and the cell suspension was added into a matrix-coated 6-well-plate containing 10% FBS and DMEM. The medium was changed 24 hours after the initial plating and then every three days.
Chemosensitivity test
The chemosensitivity test was performed according to previously described Protocol (16). In brief, primary GC cultures (2.5×103 cells) at passages 3 were plated in a 96-well-plate. After 24 hours, various concentrations (0–100 µM) of anticancer drugs (cisplatin, oxaliplatin, 5-FU and irinotecan) were added. The culture medium was changed after 48 hours and incubated until day five. The cell culture supernatant was then removed and MTT dye was added, and specimens were incubated for an additional four hours. The MTT-formazan was dissolved in DMSO, and absorbance was measured using a microplate reader at 540 nm with a reference wavelength of 630 nm. Optical density was found to be directly proportional to the number of living cells in the culture. IC50 refers to the concentration of drug required to kill 50% of the cells. The value of highly sensitive was indicated by IC50 in a range of 0 to 9 µM, moderate sensitivity was indicated by IC50 in a range of 11 to 19 µM, and resistance was indicated by IC50 of more than 20 µM. The morphology of the cells was observed under an inverted microscope.
Enzyme-linked immunosorbent assay (ELISA) for IL-6 and IL-8
ELISA was performed as previously described (15) using commercial IL-8 and IL-6 ELISA kits that were obtained from R and D Systems, Inc. (Minneapolis, MN, USA). Cells (3×105 cells per well) were plated in a six-well-plate and incubated at 37 °C for 48 hours to acquire the basal level of cytokine. To determine the effects of cisplatin and oxaliplatin treatments on ex vivo cytokine expression, non-toxic doses of cisplatin and oxaliplatin were used to treat the primary culture for 48 hours. An equal volume of cell culture supernatants was collected. The assays were performed in passages 4, 5 and 6 of the primary gastric cultures, and the concentrations of IL-6 and IL-8 in the culture supernatants were determined by comparing their optical density with the standard curve. A high expression level was determined by a level of cytokine that was higher than the mean, while low expressions were indicated by a level of cytokine lower than the mean.
Statistics
SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL, USA) and STATA 15.1 program (StataCorp LP, College Station, TX, USA) were used for statistical analyses. Non-parametric data and the correlation study between cytokine expression and the clinicopathological parameters were analyzed by Fisher’s exact test and parametric value comparison by t-test was used for mean drug sensitivity comparison before and after treatment. The results were expressed as mean ± SD. The P value <0.05 was considered statistically significant.
Results
Characteristics of primary gastric culture
The patients underwent endoscopic biopsies at Maharaj Nakorn Chiang Mai Hospital (Chiang Mai, Thailand) between June 2014 and December 2016. The age range of the patients was 25–78 years, and the ratio of males to females was 24:25. The tumor tissue samples were collected immediately following the endoscopic biopsy. All 49 patients had not received chemotherapy treatments before the endoscopic biopsy. Among them, 11 patients were classified as partial or poor responders after drug interventions with the FOLFOXIV regimen. The diagnoses were histopathologically confirmed by a senior pathologist, and there were 15 patients that were identified as poorly differentiated (non-SRC), 19 patients were identified as poorly differentiated with signet ring cells (SRC), 10 patients were identified as moderately differentiated and 5 patients were classified in the well- differentiated sub-type, respectively. Lauren classification indicated that there were 11 and 38 patients in the intestinal type and diffused type, respectively. Based on TNM classification, there were 1 patient that was identified as stage 1B, 5 patients as stage IIB, 4 patients as stage IIIA, 13 patients as stage IIIB, 5 patients as stage IIIC and 21 patients as stage IV (Figure 1 and Table 1).
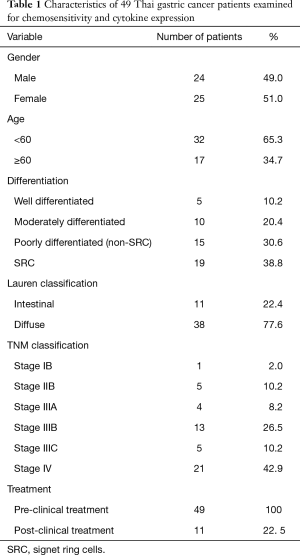
Full table
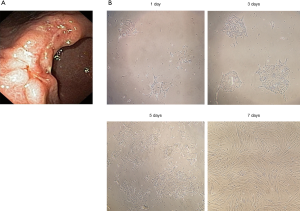
Morphology of primary gastric cultures obtained from patients
Primary cultures obtained from GC patients were established in this study within a day after sample collection as shown in Figure 2A. The colonies of GC cells were attached and spread on six-well-plates. The cells morphology of gastric primary culture in day 1, day 3 day 5 and day 7 were shown in Figure 2B. Cells were ready to be used when 80% of confluence was reached in day 7. The cells were used to determine the cytotoxic effects of anticancer drugs and to determine the levels of IL-6 and IL-8 expression.
Chemosensitivity of chemotherapeutic drugs to primary cultures obtained from pre-clinical treatment samples
This study investigated the most effective drugs to treat Thai GC patients based on the histological sub-types. In this study, we assessed the sensitivity of ex vivo primary gastric cultures obtained from 49 patients without receiving previous clinical treatments by chemotherapeutic drugs including cisplatin, oxaliplatin, 5-FU and irinotecan using MTT assay for 5 days. The degree of chemosensitivity varied with the histological type of GC (Table 2). Cisplatin was found to be cross-resistant to oxaliplatin (P=0.002). The well-differentiated culture types had a significantly higher sensitivity to platinum-based drugs (IC50 for cisplatin and oxaliplatin are 9.6±0.00 and 8.0±1.21 µM, respectively) and 5-FU (IC50 =11.6±0.28 µM) when compared to moderately and poorly differentiated culture types (P<0.05). Poorly differentiated SRC and non-SRC sub-types showed a significantly higher sensitivity to irinotecan (IC50 for non-SRC and SRC are 10.89±2.80 and 10.89±0.76 µM, respectively) when compared to the well differentiated, and moderately differentiated culture types (P<0.05). Lauren classification (Table 3) intestinal type showed significantly higher sensitivity to platinum drugs (IC50 for cisplatin and oxaliplatin are 9.44±0.91 and 8.78±0.31 µM, respectively), while the diffused type showed significantly higher sensitivity to irinotecan (IC50 =8.61±0.24 µM, P<0.05).
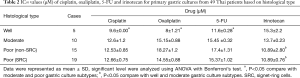
Full table

Full table
Correlation of IL-6 and IL-8 levels in 49 primary cultures with chemosensitivity to standard drugs
The correlation between basal cytokine levels and chemosensitivity of 4 drugs were investigated in 49 patient samples. There was no correlation of IL-6 and drug resistance when treatments started with each of the 4 chemotherapeutic drugs (P>0.05). More importantly, basal levels of the IL-8 levels displayed a significant level of correlation with chemosensitivity to cisplatin (P<0.001) and oxaliplatin (P<0.001) (Table 4).
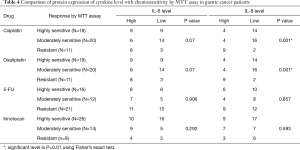
Full table
Chemosensitivity of chemotherapeutic drugs to primary cultures obtained from pre- and post-clinical treatment samples
This study investigated the sensitivity of chemotherapeutic drugs when comparing the results before and after the clinical chemotherapeutic 6-cycle treatments. Sub-group analysis was done involving 11 partial or poor responder patients who underwent endoscopic biopsies before and after clinical chemotherapeutic treatment by FOLFOXIV. The results are shown in Figure 3. Most of the patients tended to be more resistant to the platinum-based drugs by increasing IC50 levels to the drug. Additionally, 6 of 11 patients showed significant increases of IC50 to cisplatin (P<0.05) (Figure 3A), whereas, 5 of 11 patients showed significant increases of IC50 to oxaliplatin (P<0.05) (Figure 3B). IC50 to 5-FU showed significant increases in patients No. 3 and No. 5 (Figure 3C). Among them, only patient No. 4 showed significant decreases in IC50 to platinum drugs (cisplatin and oxaliplatin) and 5-FU, while 4 of 11 patients showed significant increases in IC50 values to irinotecan (Figure 3D). The statistical analysis was performed before and after the clinical treatment.
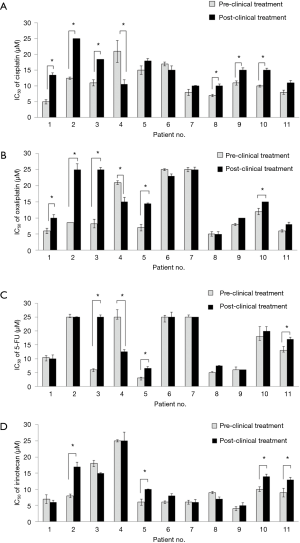
Table 5 shows the mean of the IC50 levels of the drug treatments administered in pre-clinical treatments and post-clinical treatments. The mean levels of IC50 of cisplatin in pre-clinical and post-clinical treatments were 11.40±1.42 and 14.68±1.37 µM, respectively. The mean levels of IC50 of oxaliplatin in pre-clinical and post-clinical treatments were 11.97±2.34 and 16.86±2.38 µM, respectively. IC50 levels of cisplatin and oxaliplatin in post-clinical chemotherapy treatments were significantly higher than in the pre-clinical chemotherapy treatments at P=0.049 and P=0.014, respectively. There were no significant differences observed between the IC50 levels of 5-FU and irinotecan in the pre-clinical and post-clinical treatments.
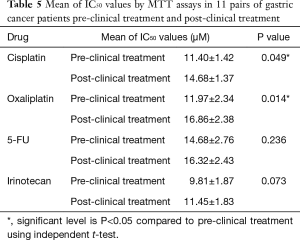
Full table
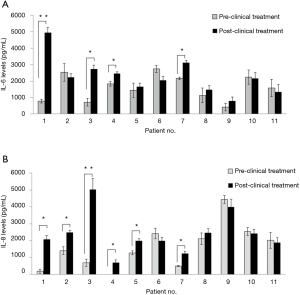
IL-6 and IL-8 levels in primary gastric cultures obtained from pre-clinical treatment and post-clinical treatment samples
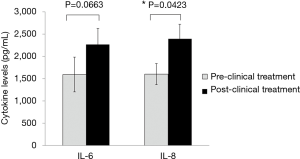
Cytokine levels were investigated in primary gastric cultures obtained from endoscopic biopsies before and after clinical chemotherapeutic treatments (Figure 4). After 6 cycles of chemotherapy, patients received a repeat endoscopy examination with a biopsy to ex vivo secondary drug tests with cytokine levels being measured again. Notably, 4 of 11 patients showed significant increases in IL-6 levels after clinical drug treatments (Figure 4A), while 6 of 11 patients showed significant increases in basal IL-8 levels (P<0.05) (Figure 4B). The means of the IL-6 and IL-8 expression levels measured by ELISA assay study in GC patients were compared with the pre-clinical and post-clinical treatments in 11 primary cultures obtained from Thai GC patients. The mean levels of IL-6 protein expressions in pre- clinical and post-clinical treatments were 1,595.41±389.92 and 2,268.27±361.52 pg/mL, respectively. The mean levels of IL-8 protein expression in pre-clinical and post-clinical treatments were 1,600±234.61 and 2,390±332.90 pg/mL, respectively. IL-8 protein expression in post-clinical chemotherapy treatments was significantly higher than in the pre-clinical chemotherapy or in the up-regulation with P=0.0423. Notably, there was no correlation of IL-6 (P=0.0663) (Figure 5).
Effect of platinum-based drug treatment on ex vivo cytokine expression
The effects of clinical drug treatments on cytokine expression in patients were mimicked by treating 49 primary cultures obtained from pre-treated patients who were treated with the chemotherapeutic drug ex vivo. Platinum-based drugs cisplatin and oxaliplatin were used in this experiment as surrogates of the first-line drugs. Patients undergoing pre-clinical treatment biopsies were studied after drug treatments (cisplatin and oxaliplatin) using non-toxic doses by MTT assay. The mean levels of IL-6 protein expressions in the non-treatment group, the cisplatin treatment group, and the oxaliplatin treatment group were 1,752.1, 1,584.0 and 1,505.9 pg/mL, respectively. The mean levels of IL-8 protein expressions in the non-treatment, cisplatin treatment and oxaliplatin treatment groups were 1,382.9, 1,245.6 and 1,152.9 pg/mL, respectively. The oxaliplatin treatment showed decreases in levels of IL-6 and IL-8 protein expression with P=0.059 and P=0.046 when compared to the non- treatment group. The above results could imply that the cell culture obtained from GC patients who showed positive responses to ex vivo chemotherapy treatments displayed significantly declined cytokine levels with findings coinciding with the patients who had revealed clinical outcomes.
Discussion
Our previous study has shown that high levels of IL-8 mRNA expression were detected in more than 80% of Thai GC patients (17). These findings have led to our hypothesis that IL-8 and/or another cytokine might be candidates for predictive biomarkers in monitoring drug resistance in GC patients. Presently, there is limited available information, and few research studies have been conducted regarding GC cases among the Thai population. Moreover, there is no available evidence, nor has there been any translational research that has been conducted, on the IL-8 and IL-6 protein expression levels in correlation to chemosensitivity to first line or second line drugs treatment in primary gastric cultures obtained from patients. Another report has shown the relationship between the circulating levels of cytokines such as IL-8 and GC progression as its significant involvement in patient with late stage (IV) GCs (11). In the context of chemotherapeutic drug resistance and cytokine expression in GC, previous studies found that, oxaliplatin treatment induced IL-8 secretion in acquired resistant colorectal cancer cells via the activation of Akt/NF-κB signaling pathways. Transcriptomic profiling revealed the up-regulation of three NF-κB regulated CXC-chemokines, CXCL8 (or IL-8), CXCL1 and CXCL2, in the resistant cells. Moreover, inhibition of these pathways could increase oxaliplatin sensitivity (18,19). The study in metastatic prostate cancer cells demonstrated the increasing of IL-8 expression and secretion upon Oxaliplatin treatment via NF-κB activation which correlated with drug resistance (20). Therefore, it could be suggested that, platinum-based chemotherapeutic drugs are able to induce cytokine secretion, and eventually lead to drug resistance. Herein, our data coincided with previous report and demonstrated significant decrease in drug sensitivity to cisplatin and oxaliplatin, meanwhile IL-8 protein expression levels were significantly increase in both drug treatments. These results were recorded after patients were exposed to 6 cycles of the platinum drug. The platinum drug-resistant mechanisms were developed when the cycles were increased, while cytokine expression levels were also remarkably increased.
The diffused type of gastric adenocarcinoma revealed the highest incidence of the sub-groups at 77.6 percent (Table 1) among Thai GC patients in northern Thailand. Interestingly, drug sensitivity tests showed higher sensitivity to platinum drugs in the intestinal type and higher sensitivity to irinotecan in the diffused type (Table 3). Similar to our study, the S-1 and Taxotere (docetaxel) therapy for advanced GC Randomized phase III Trial (START) study revealed that S-1, irinotecan and docetaxel exhibit a certain advantage in the treatment of diffuse GC (21). Based on the WHO classification, the poorly differentiated type with the SRC was the most frequent sub-type among the northern Thai population (38.8%) followed by the non- SRC sub-type (30.6%), whereas the moderately differentiated and well differentiated sub-types were recorded at 20.4% and 10.2%, respectively (Table 1). Investigations involving the drug sensitivity test by histological type showed higher levels of sensitivity to platinum drugs in well differentiated types when compared to the moderately and poorly differentiated types, respectively (Table 3). Regarding SRCs, the poorly differentiated type showed higher sensitivity to irinotecan when compared to the moderately and well differentiated types. These observations are generally consistent with the clinical response behavior of these types of cancers in terms of drug response and cancer prognosis.
We found a correlation between the chemosensitivity test by and basal levels of IL-8. Patients with low levels of IL-8 showed more sensitivity to the platinum drugs cisplatin and oxaliplatin when compared to patients with high IL-8 levels (Table 4). Recent studies have shown a correlation between IL-8 levels and chemoresistance to the platinum drug oxaliplatin in the GC cell line (14), which has demonstrated that overexpression of IL-8 promotes the adhesion, migration, invasion and chemoresistance of GC using human GC cell lines (MKN-45 and KATO-III). Another study has found that circulating levels of IL-8 may play a role in H-pylori-associated gastric carcinogenesis in northern India (22). Our findings are the first to report on the cytokine IL-8 levels in the primary cultures obtained from tissue samples in relation to sensitivity to platinum drugs including oxaliplatin and cisplatin. Cytokine IL-6 protein expression levels also increased after clinical chemotherapeutic treatment. On the other hand, when ex vivo treatment with the platinum drugs cisplatin and oxaliplatin in 49 primary cultures were performed. Surprisingly, the mean averages of the IL-6 and IL-8 levels decreased after short-term drug treatments. We assume that the primary population members, who responded well to cisplatin and oxaliplatin in the ex vivo treatments, had a tendency to display declining IL-8 levels after treatment. However, the survival may have major first effect by drug sensitivity. The clinicians in this study can see the trend of 11 patients that 4 patients who have longer survival, and its result in stable or declination of IL-8, and first drug clinical response to chemotherapy treatment.
Taken together, this is a preliminary study regarding cytokine levels and chemotherapeutic sensitivity in Thai GC patients and is a pilot clinical observatory data result in linkage with drug resistant or response in terms of biomarker interpretation in clinical mean. The data imply that upregulation of IL-8 after drug intervention might be useful as predictive biomarker in monitoring drug resistance in GC patients. Further experimental studies on GC patients may be worthwhile in order to discern a greater level of versatility in a clinical setting. Moreover, this study was a newly designed pilot study that was focused only on the northern Thai ethnic population. Ethnic differences or comparative studies should be applied in other population-based study designs, and larger sample sizes should be applied to improve upon the limitations of this study.
Acknowledgments
We thank Associate Professor Nirush Lertprasertsuk, MD, PhD. for the stress review of the medical records and the secondary pathology review.
Funding: This work was supported by Chiang Mai University and grants from the National Research Council of Thailand (NRCT, 2559A10403034), and the Faculty of Medicine, Chiang Mai University (Grant No.096/2560), Center for Research and Development of Natural Products for Health.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Chiang Mai University Ethics Committee for Human Research. The ethics number and the study code number are 408/2015 and SUR-2558-03015, respectively with written informed consent being obtained prior to use of the endoscopic biopsy samples.
References
- Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer 2011;11:135-40. [Crossref] [PubMed]
- Uchida T, Miftahussurur M, Pittayanon R, et al. Helicobacter pylori Infection in Thailand: A Nationwide Study of the CagA Phenotype. PLoS One 2015;10:e0136775. [Crossref] [PubMed]
- Chon SH, Berlth F, Plum PS, et al. Gastric cancer treatment in the world: Germany. Transl Gastroenterol Hepatol 2017;2:53. [Crossref] [PubMed]
- Dranoff G. Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 2004;4:11-22. [Crossref] [PubMed]
- Lippitz BE. Cytokine patterns in patients with cancer: a systematic review. Lancet Oncol 2013;14:e218-28. [Crossref] [PubMed]
- Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment. J Immunol Res 2014;2014:149185. [Crossref] [PubMed]
- Chen J, Huang XF. Interleukin-6 promotes carcinogenesis through multiple signal pathways. Comment on: Clinical significance of interleukin-6 gene polymorphism and IL-6 serum level in pancreatic adenocarcinoma and chronic pancreatitis. Dig Dis Sci 2009;54:1373-4. [Crossref] [PubMed]
- Ashizawa T, Okada R, Suzuki Y, et al. Clinical significance of interleukin-6 (IL-6) in the spread of gastric cancer: role of IL-6 as a prognostic factor. Gastric Cancer 2005;8:124-31. [Crossref] [PubMed]
- Wu X, Tao P, Zhou Q, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget 2017;8:20741-50. [PubMed]
- Chung HW, Lim JB. High-mobility group box-1 contributes tumor angiogenesis under interleukin-8 mediation during gastric cancer progression. Cancer Sci 2017;108:1594-601. [Crossref] [PubMed]
- Sánchez-Zauco N, Torres J, Gómez A, et al. Circulating blood levels of IL-6, IFN-gamma, and IL-10 as potential diagnostic biomarkers in gastric cancer: a controlled study. BMC Cancer 2017;17:384-93. [Crossref] [PubMed]
- Yamada S, Kato S, Matsuhisa T, et al. Predominant mucosal IL-8 mRNA expression in non-cagA Thais is risk for gastric cancer. World J Gastroenterol 2013;19:2941-9. [Crossref] [PubMed]
- Park SY, Han J, Kim JB, et al. Interleukin-8 is related to poor chemotherapeutic response and tumourigenicity in hepatocellular carcinoma. Eur J Cancer 2014;50:341-50. [Crossref] [PubMed]
- Kuai WX, Wang Q, Yang XZ, et al. Interleukin-8 associates with adhesion, migration, invasion and chemosensitivity of human gastric cancer cells. World J Gastroenterol 2012;18:979-85. [Crossref] [PubMed]
- Wongsirisin P, Yodkeeree S, Limpakan S, et al. Curcumin inhibition of the effects of Tip α induced cytokine expression in gastric cancer patients. Pharma Nutrition 2018;6:100-6. [Crossref]
- Wongsirisin P, Yamada SL, Yodkeeree S, et al. Association of DNA repair and drug transporter in relation to chemosensitivity in primary culture of Thai gastric cancer patients. Biol Pharm Bull 2018;41:360-7. [Crossref] [PubMed]
- Chongruksut W, Limpakan Yamada S, Chakrabandhu B, et al. Correlation of Helicobacter pylori and interleukin-8 mRNA expression in high risk gastric cancer population prediction. World J Gastrointest Oncol 2016;8:215-21. [Crossref] [PubMed]
- Dabkeviciene D, Jonusiene V, Zitkute V, et al. The role of interleukin-8 (CXCL8) and CXCR2 in acquired chemoresistance of human colorectal carcinoma cells HCT116. Med Oncol 2015;32:258. [Crossref] [PubMed]
- Ruiz de Porras V, Bystrup S, Martínez-Cardús A, et al. Curcumin mediates oxaliplatin-acquired resistance reversion in colorectal cancer cell lines through modulation of CXC-Chemokine/NF-κB signalling pathway. Sci Rep 2016;6:24675. [Crossref] [PubMed]
- Wilson C, Purcell C, Seaton A, et al. Chemotherapy-induced CXC-chemokine/CXC-chemokine receptor signaling in metastatic prostate cancer cells confers resistance to oxaliplatin through potentiation of nuclear factor-κB transcription and evasion of apoptosis. J Pharmacol Exp Ther 2008;327:746-59. [Crossref] [PubMed]
- Koizumi W, Kim YH, Fujii M, et al. JACCRO and KCSG Study Group. Addition of docetaxel to S-1 without platinum prolongs survival of patients with advanced gastric cancer: A randomized study (START). J Cancer Res Clin Oncol 2014;140:319-28. [Crossref] [PubMed]
- Kumar S, Kumari N, Mittal RD, et al. Association between pro-(IL-8) and anti-inflammatory (IL-10) cytokine variants and their serum levels and H. pylori-related gastric carcinogenesis in northern India. Meta Gene 2015;6:9-16. [Crossref] [PubMed]